Direct measurement of anatomic regurgitant orifice area (AROA) by 3-dimensional transesophageal echocardiography was evaluated for analysis of mitral regurgitation (MR) severity. In 72 patients (age 70.6 ± 13.3 years, 37 men) with mild to severe MR, 3-dimensional transesophageal echocardiography and transthoracic color Doppler echocardiography were performed to determine AROA by direct planimetry, effective regurgitant orifice area (EROA) by proximal convergence method, and vena contracta area (VCA) by 2-dimensional color Doppler echocardiography. AROA was measured with commercially available software (QLAB, Philips Medical Systems, Andover, Massachusetts) after adjusting the first and second planes to reveal the smallest orifice in the third plane where planimetry could take place. AROA was classified as circular or noncircular by calculating the ratio of the medial-lateral distance above the anterior-posterior distance (≤1.5 compared to >1.5). AROA determined by direct planimetry was 0.30 ± 0.20 cm 2 , EROA determined by proximal convergence method was 0.30 ± 0.20 cm 2 , and VCA was 0.33 ± 0.23 cm 2 . Correlation between AROA and EROA (r = 0.96, SEE 0.058 cm 2 ) and between AROA and VCA (r = 0.89, SEE 0.105 cm 2 ) was high considering all patients. In patients with a circular regurgitation orifice area (n = 14) the correlation between AROA and EROA was better (r = 0.99, SEE 0.036 cm 2 ) compared to patients with noncircular regurgitation orifice area (n = 58, r = 0.94, SEE 0.061 cm 2 ). Correlation between AROA and EROA was higher in an EROA ≥0.2 cm 2 (r = 0.95) than in an EROA <0.2 cm 2 (r = 0.60). In conclusion, direct measurement of MR AROA correlates well with EROA by proximal convergence method and VCA. Agreement between methods is better for patients with a circular regurgitation orifice area than in patients with a noncircular regurgitation orifice area.
Direct planimetry of anatomic regurgitation orifice area (AROA) by real-time 3-dimensiosonal transesophageal echocardiography (RT 3D TEE) was evaluated to quantify mitral regurgitation (MR) severity. Measurements of AROA by 3D TEE were compared to those of effective regurgitant orifice area (EROA) obtained by the proximal flow convergence or proximal isovelocity surface area method and vena contracta area (VCA) determined from color Doppler echocardiography.
Methods
We enrolled 75 consecutive patients with 1 MR to ≥4 MRs defined by routine color Doppler echocardiography. Patients with significant mitral stenosis (mitral valve area <2.0 cm 2 ), mitral prosthesis, or irregular rhythm were not included. Due to impaired 2-dimensional transesophageal echocardiographic image quality, 3 patients were excluded. In the remaining 72 patients (96%) transthoracic color Doppler echocardiogram and 2-dimensional and RT 3D transesophageal echocardiogram could be obtained. This study was approved by the institutional review board of the University Clinic Aachen (Aachen, Germany) and all patients provided written informed consent. Echocardiographic studies were performed with a commercially available echocardiographic system (iE 33, Philips Medical Systems, Andover, Massachusetts) and transesophageal echocardiographic probe (X7-2t) allowing real-time 3D TEE.
Transesophageal color Doppler flow imaging was performed using a multiplane transesophageal echocardiographic probe in midesophageal position with a scanning plane from 30° to 60° to achieve an intercommissural view of the mitral valve and a scanning plane from 120° to 150° to achieve a left ventricular outflow tract view. The narrowest sector angle that allowed visualization of the MR VC was used to maximize color flow imaging frame rate. Nyquist velocity ranged from 39 to 70 cm/s. The high-velocity core of the jet was evaluated to define the VC. In each patient, a sweep of the mitral valve coaptation line was performed with the transesophageal probe in the 2 views to find the location with the largest regurgitation orifice and optimize visualization of the area of proximal flow acceleration, the VC, and the downstream expansion of the jet. For each echocardiographic window, zoom mode was used to optimize visualization and measurement of the VC. VC was measured in each view from the systolic frame showing the largest diameter of a clearly defined VC. For each view, an average of 3 beats was taken. To account for the possibility of nonsymmetrical orifices, a biplane VC width was calculated by averaging VC measurements from the 2 roughly orthogonal planes. The VC regurgitant area was calculated as VCA = π(VC biplane/2) 2 .
Appearance of the proximal convergence field was optimized by baseline shifting of the color Doppler aliasing velocity to 30 to 39 cm/s. Radial distance between the first aliasing contour (red/blue interface) and the center of the regurgitant orifice was measured at the time of the largest convergence image. For patients with nonflail mitral leaflets, the orifice was assumed to be at the plane passing through the tips of the mitral leaflets; for flail mitral leaflets, the orifice was assumed to lie in the plane of the nonflail leaflet.
Maximal instantaneous regurgitant flow was calculated as 2πr 2 v a , where r is the maximal distance to the contour of aliasing velocity v a with a hemispheric contour assumed. EROA was obtained by dividing maximal instantaneous regurgitant flow by peak regurgitant velocity obtained by continuous-wave Doppler.
RT 3D transesophageal echocardiographic studies included (1) zoom mode and (2) full-volume wide-angle acquisition with and without color flow imaging of the mitral valve. After finishing 2-dimensional transesophageal examination a full-volume wide-angle acquisition was performed to visualize mitral leaflets to identify the structure and cause of MR and locate the MR orifice area with visualization of jet direction into left atrium with color flow imaging. In addition, 3D zoom mode was used to acquire a volume with the narrowest possible depth and lateral and elevation width focused on the coaptation line of mitral valve leaflets to achieve volume rates of 10 to 20 frames/s with medium to high density. AROA was determined off-line from 3D zoom volumes with dedicated analysis software (3DQ, QLAB 7.0, Philips Medical Systems). After roughly adjusting the first plane parallel and the second plane perpendicular to the intercommissural line of the mitral valve along the apex of the left ventricle in 3D overview, intercommissural and left ventricular outflow tract views were generated. Then, the 2 planes were moved parallel to the assumed location of the MR orifice in each frame mid- to end-systole and then rotated along the assumed regurgitation jet direction. The third plane was positioned orthogonal to the 2 previous planes. To obtain the smallest orifice this plane was placed across the coaptation line where direct planimetry of the regurgitation orifice area could be performed ( Figure 1 ). AROA was classified as circular or noncircular by calculating the ratio of the anterior-posterior distance versus the medial-lateral distance. Circular AROA was defined as a ratio ≤1.5 and noncircular AROA as a ratio >1.5.
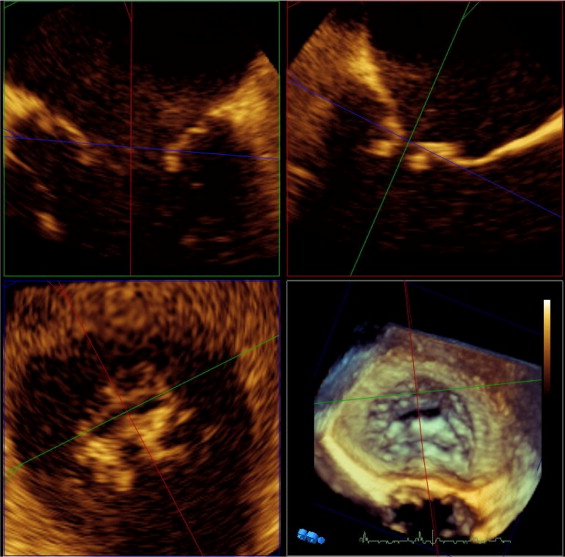
The 3 techniques to determine regurgitant orifice area were evaluated independently by 3 different observers. Each observer obtained only the imaging set of a technique and was blinded to results of the other 2 techniques.
In 26 randomly selected studies, 2 observers independently measured the AROA, and interobserver agreement was assessed by linear regression and Bland-Altman analysis. These same studies were also reexamined by 1 observer at a separate time 1 month later to determine intraobserver agreement.
Statistical analysis was performed with a special statistical analysis program (MedCalc 9.5.1.0, MedCalc, Mariakerke, Belgium). Continuous data are presented as mean ± SD, Pearson correlation coefficient with p value, and Pearson correlation coefficient with 95% confidence interval. A p value <0.05 was considered statistically significant. AROA as determined by RT 3D TEE was compared to EROA from proximal isovelocity surface area and VCA as determined by color Doppler echocardiography by linear regression analysis and Bland-Altman analysis of agreement for the entire population, for circular and noncircular AROAs, for EROAs <0.2 and ≥0.2 cm 2 , and intra- and interobserver variabilities.
Results
Clinical characteristics of the 72 patients with 1 MR to ≥4 MRs are listed in Table 1 .
| Age (years) | 71 ± 13 |
| Men/women | 37/35 |
| New York Heart Association class ⁎ | |
| I | 9 (13%) |
| II | 26 (36%) |
| III | 28 (38%) |
| IV | 9 (13%) |
| Ejection fraction (%) | 48.9 ± 13.6 |
| 4-chamber left atrial area (cm 2 ) | 25.0 ± 5.8 |
| 4-chamber color Doppler jet area (cm 2 ) | 7.4 ± 3.4 |
| Cause of mitral regurgitation | |
| Ischemic heart disease/dilated cardiomyopathy | 22 (30%) |
| Mitral valve prolapse/flail leaflet | 25 (35%) |
| Rheumatic heart disease/others | 25 (35%) |
| Regurgitant volume by convergence method (ml) | 48.2 ± 32.8 |
⁎ New York Heart Association functional classification for severity of heart failure.
Most MRs were due to degenerative changes of mitral leaflets, although functional MR due to impaired left ventricular function with dilatation of the mitral ring was another common cause.
Regurgitant orifice areas determined by the 3 techniques are listed in Table 2 . Regurgitant orifice area determined by VC analysis was greater with 0.33 ± 0.23 compared to 0.30 ± 0.20 cm 2 defined by planimetry of AROA on RT 3D transesophageal echocardiogram and by flow convergence technique. There was high agreement between AROA determined by planimetry and EROA determined by convergence method for the entire population (r = 0.962, SEE 0.058 cm 2 ; Figures 2 and 3 ).
| EROA (cm 2 ) | AROA (cm 2 ) | VCA (cm 2 ) | |
|---|---|---|---|
| All patients (n = 72) | 0.30 ± 0.20 | 0.30 ± 0.20 | 0.33 ± 0.23 |
| Circular anatomic regurgitation orifice area (n = 14) | 0.33 ± 0.25 | 0.32 ± 0.24 | 0.34 ± 0.24 |
| Noncircular anatomic regurgitation orifice area (n = 58) | 0.30 ± 0.19 | 0.29 ± 0.20 | 0.32 ± 0.22 |
Using RT 3D TEE to define the shape of the AROA, sphericity index as the ratio of maximal diameter to minimal diameter of regurgitant orifice area ranged from 1.1 to 8.9. In 14 patients (19%) the ratio of maximal diameter to minimal diameter was ≤1.5 and in 58 patients (81%) the ratio was >1.5, indicating a noncircular regurgitant orifice area to be predominant. When looking at agreement between AROA and EROA depending on shape of AROA defined by RT 3D TEE, there was slightly greater agreement in the circular AROA group (r = 0.993, SEE 0.036 cm 2 ) than in the noncircular AROA group (r = 0.952, SEE 0.061 cm 2 ; Figure 4 ).




