There are limited data on the impact of diabetes mellitus (DM) on the risk of subclinical atherosclerosis. Therefore, we sought to investigate the impact of DM on the risk of subclinical atherosclerosis in asymptomatic subjects. We analyzed 2,034 propensity score–matched asymptomatic subjects who underwent coronary computed tomographic angiography (mean age 55.9 ± 8.2 years; men 1,725 [84.8%]). Coronary artery calcium score, degree and extent of coronary artery disease (CAD), and clinical outcomes were assessed. High-risk CAD was defined as at least 2-vessel coronary disease with proximal left anterior descending artery involvement, 3-vessel disease, or left main disease. Compared with subjects without DM, those matched with DM had higher coronary artery calcium score (89.9 ± 240.4 vs 62.8 ± 179.5, p = 0.004) and more significant CAD (≥50% diameter stenosis, 15.2% vs 10.2%, p = 0.001), largely in the form of 1-vessel disease (10.8% vs 7.3%, p = 0.007). However, there were no significant differences between matched pairs in significant CAD in the left main or proximal left anterior descending artery (5.3% vs 3.8%, p = 0.138), multivessel disease (4.4% vs 2.9%, p = 0.101), and high-risk CAD (4.3% vs 2.7%, p = 0.058). During the follow-up period (median 21.8, interquartile range 15.2 to 33.4 months), there was no significant difference in the composite of all-cause death, myocardial infarction, acute coronary syndrome, and coronary revascularization between 2 groups (hazard ratio 1.438, 95% confidence interval 0.844 to 2.449, p = 0.181). In asymptomatic subjects, those matched with DM have more subclinical atherosclerosis, mainly confined to non–high-risk CAD, than those matched without DM, and there are no differences in high-risk CAD and clinical outcomes.
Over the past decades, the prevalence of diabetes mellitus (DM) has increased rapidly in most countries, and DM has become a major public health concern. Coronary artery disease (CAD) is the leading cause of death in patients with DM, and DM is associated with an increased prevalence of atherosclerosis. However, data on the impact of DM on the risk of subclinical atherosclerosis in asymptomatic subjects are limited. Recently, with the advent of multidetector row computed tomography, coronary computed tomography angiography (CCTA) has proved effective in providing comprehensive information on coronary atherosclerosis, including lesion location, severity, and the characteristics of atherosclerotic plaques. Therefore, we investigated the influence of DM on the risk of subclinical atherosclerosis and clinical outcomes using propensity-matched groups in a large cohort of asymptomatic Korean subjects who underwent CCTA.
Methods
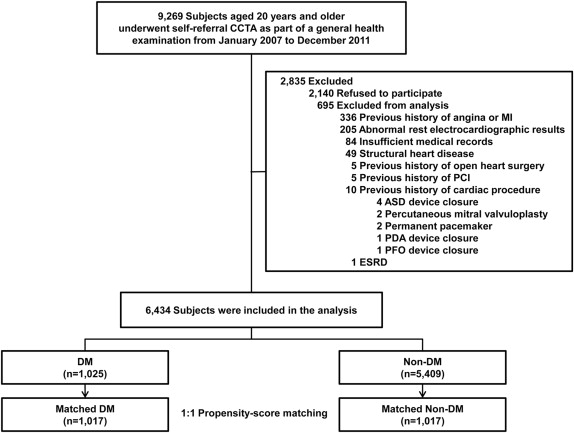
From January 2007 to December 2011, we enrolled 9,269 consecutive South Korean subjects aged ≥20 years who had undergone self-referral CCTA evaluation as part of a general health examination in the Health Screening and Promotion Center at the Asan Medical Center. All were made aware of the possible risks associated with CCTA and provided informed consent. A total of 7,129 subjects (76.9%) consented to participate in the study. DM was defined as a fasting plasma glucose concentration ≥126 mg/dl or a self-reported history of DM and/or treatment by dietary modification or use of antidiabetic medication. We excluded subjects with (1) a history of angina or myocardial infarction (MI); (2) abnormal rest electrocardiographic results, that is, pathological Q waves, ischemic ST segments, or T-wave changes, or left bundle branch blocks; (3) insufficient medical records; (4) structural heart disease; (5) a history of open-heart surgery or percutaneous coronary intervention (PCI); (6) a previous cardiac procedure; or (7) renal insufficiency (creatinine >1.5 mg/dl). Finally, 6,434 subjects were enrolled ( Figure 1 ). The study was approved by the local Institutional Review Board of the Asan Medical Center (Seoul, Korea). All subjects provided written informed consent.
Basic demographic data for the subjects were acquired from a database maintained by the Health Screening and Promotion Center at the Asan Medical Center. Medical histories of angina, MI, stroke, structural heart disease, open-heart surgery, PCI, previous cardiac procedure, DM, hypertension, or hyperlipidemia; family histories of CAD; and smoking status were collected from a systematic questionnaire answered before a general health examination. In the general health examination, height, body weight, body mass index, waist circumference, and blood pressure were measured. In addition, fasting plasma glucose, glycated hemoglobin, uric acid, creatinine, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglyceride, and high-sensitivity C-reactive protein concentrations were measured on the day of the examination, after a fast of ≥12 hours. A 10-year CAD risk score was calculated using the Framingham risk model. With regard to the Framingham risk score, study participants were categorized as having either a low (<10%), intermediate (10% to 20%), or high (>20%) 10-year risk for CAD.
CCTA was conducted using either single-source 64-slice CT (LightSpeed VCT; GE, Milwaukee, Wisconsin) or dual-source CT (Somatom Definition; Siemens, Erlangen, Germany). Patients with no contraindication to β-adrenergic blocking agents and with initial heart rates >65 beats/min received an oral dose of 2.5 mg bisoprolol (Concor; Merck, Darmstadt, Germany) 1 hour before the CT examination. CT scanning was performed in the prospective ECG-triggering mode or the retrospective ECG-gating mode with ECG-based tube current modulation. Two puffs (2.5 mg) of isosorbidedinitrate (Isoket spray; Schwarz Pharma, Monheim, Germany) were sprayed into the patient’s oral cavity before contrast injection. During CCTA acquisition, 60 to 80 ml of iodinated contrast (Iomeron 400; Bracco, Milan, Italy) was injected at 4 ml/s, followed by a 40-ml saline flush. A region of interest was placed in the ascending aorta, and image acquisition was automatically initiated once a selected threshold (100 HU) had been reached using bolus tracking. A standard scanning protocol was used, and the tube voltage and tube current-time product were adjusted according to the patient’s body size as follows: 100 or 120 kVp tube voltage, 240 to 400 mAs per rotation (dual-source CT), and 400 to 800 mA (64-slice CT) tube current.
All CCTA scans were analyzed using a dedicated workstation (Advantage Workstation, GE; or Volume Wizard, Siemens) by experienced cardiovascular radiologists (DHY, T-HL, and J-WK). According to the guidelines of the Society of Cardiovascular Computed Tomography, a 16-segment coronary artery tree model was used. A coronary artery calcium score (CACS) was measured as described. Plaques were defined as structures >1 mm 2 within and/or adjacent to the vessel lumen, which could be clearly distinguished from the lumen and surrounding pericardial tissue. Plaques containing calcified tissue involving >50% of the plaque area (density >130 HU) were classified as calcified, plaques with <50% calcium were classified as mixed, and plaques without calcium were classified as noncalcified. The percentage of the average diameter stenosis was evaluated, and the severity of the stenosis was graded as normal (no plaque), mild (<50%), moderate (50% to 69%), or severe (≥70%). Stenosis ≥50% was defined as significant. In addition, the overall plaque burden was determined from coronary artery plaque scores calculated from modified Duke prognostic scores, segment stenosis scores, and segment involvement scores, as described. Based on the Duke CAD index developed previously, patients were categorized as having high-risk CAD defined as at least 2-vessel coronary disease with proximal left anterior descending (LAD) artery involvement, 3-vessel disease, or left main (LM) disease.
Follow-up clinical data were obtained by review of medical records at the end of August 2012. Cardiac events were defined as a composite of all-cause death, nonfatal MI, acute coronary syndrome requiring hospitalization, or coronary revascularization. Deaths were identified using identification numbers, which were assigned to the subjects at birth certificates, in the National Statistical Office. The diagnosis of MI was based on the presence of new Q waves in at least 2 contiguous leads or an elevation of creatine kinase or its MB isoenzyme to at least 3 times the upper limit of the normal range at follow-up. Revascularization was performed if there were stenosis of at least 50% of the diameter on invasive coronary angiography with a positive stress test or if there was a stenosis of at least 70% on invasive coronary angiography.
Categorical data were compared using the chi-square statistics or Fisher’s exact test and presented as frequencies. Continuous variables were analyzed using the unpaired Student’s t test and presented as the mean ± SD. To reduce potential confounding factors in this observational study, we performed an adjustment for significant differences in the 19 baseline clinical characteristics of patients using propensity score matching (for age, gender, body mass index, waist circumference, systolic and diastolic blood pressures, hypertension, hyperlipidemia, current smoking, previous stroke, family history of CAD, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglyceride, creatinine, ejection fraction, uric acid, and high-sensitivity C-reactive protein ≥2 mg/dl). After all the propensity score matches were performed, we examined the similarity of the baseline clinical variables between 2 groups by calculating standardized differences for each variables. All the standardized differences for each of the baseline variables were <0.10 (10%). Also the variables were compared between the 2 groups with the paired t test or the Wilcoxon signed-rank test for continuous variables and the McNemar’s test or marginal homogeneity test for categorical variables. In the propensity score–matched cohort, the risks of clinical events were compared using Cox regression model. Subgroup analyses were also conducted in matched subjects with DM and without DM with low and intermediate risk. All reported p values are 2 sided, and p values <0.05 were considered statistically significant. Data management and statistical analyses were conducted using SAS, version 9.1 (SAS Institute Inc., Cary, North Carolina).
Results
The baseline characteristics of the study population according to DM are listed in Table 1 . Of the study participants, 1,025 (15.9%) had DM. Subjects with DM were older and had more co-morbid conditions than those without. After propensity score matching, there were 1,017 matched pairs ( Table 2 ). The mean age of the matched subjects was 55.9 ± 8.2 years, and 1,725 participants (84.8%) were men. In matched subjects with DM, mean fasting plasma glucose was 135.5 ± 33.0 mg/dl and mean hemoglobin A1C was 6.8 ± 1.3%. In matched cohort, there were no other significant differences for any of the covariates between the DM and non-DM groups.
| Characteristics | Overall (n=6,434) | Diabetes Mellitus | P-value | |
|---|---|---|---|---|
| Yes (n=1,025) | No (n=5,409) | |||
| Age (years) | 53.7±7.6 | 55.7±7.5 | 53.3±7.5 | <0.001 |
| Men | 4,694 (73.0%) | 862 (84.1%) | 3,832 (70.8%) | <0.001 |
| Body mass index (kg/m 2 ) | 24.6±2.9 | 25.4±3.0 | 24.5±2.9 | <0.001 |
| Waist circumference (cm) | 85.9±8.3 | 88.8±7.9 | 85.3±8.3 | <0.001 |
| Systolic blood pressure (mmHg) | 120.1±13.1 | 123.3±13.6 | 119.5±12.9 | <0.001 |
| Diastolic blood pressure (mmHg) | 76.9±10.4 | 78.2±10.1 | 76.6±10.4 | <0.001 |
| Hypertension ∗ | 2,352 (36.6%) | 534 (52.1%) | 1,818 (33.6%) | <0.001 |
| Hyperlipidemia † | 2,003 (31.1%) | 454 (44.3%) | 1,549 (28.6%) | <0.001 |
| Current smoker | 1,525 (23.7%) | 316 (30.8%) | 1,209 (22.4%) | <0.001 |
| Previous stroke | 56 (0.9%) | 15 (1.5%) | 41 (0.8%) | 0.026 |
| Family history of coronary artery disease ‡ | 983 (15.3%) | 129 (12.6%) | 854 (15.8%) | 0.009 |
| Total cholesterol (mg/dL) | 195.5±34.3 | 186.4±39.0 | 197.2±33.1 | <0.001 |
| Low-density lipoprotein cholesterol (mg/dL) | 121.3±30.1 | 112.5±33.1 | 123.0±29.2 | <0.001 |
| High-density lipoprotein cholesterol (mg/dL) | 53.4±13.5 | 50.5±12.2 | 54.0±13.7 | <0.001 |
| Triglyceride (mg/dL) | 134.1±85.7 | 159.7±118.5 | 129.3±77.0 | <0.001 |
| Fasting blood glucose (mg/dL) | 104.9±20.8 | 135.8±33.2 | 99.0±9.5 | <0.001 |
| Glycated hemoglobin (%) | 5.7±0.8 | 6.9±1.3 | 5.5±0.4 | <0.001 |
| Creatinine (mg/dL) | 0.9±0.2 | 0.9±0.2 | 0.9±0.2 | 0.125 |
| Uric acid (mg/dL) | 5.6±1.4 | 5.6±1.3 | 5.6±1.4 | 0.241 |
| High-sensitivity C-reactive protein ≥2 mg/dL | 58 (0.9%) | 15 (1.5%) | 43 (0.8%) | 0.038 |
| Ejection fraction (%) | 63.3±4.1 | 63.4±4.2 | 63.3±4.1 | 0.425 |
| Framingham risk score | 7.9±5.4 | 12.3±6.0 | 7.1±4.8 | <0.001 |
| Framingham risk stratification | <0.001 | |||
| Low-risk group | 4,573 (71.1%) | 421 (41.1%) | 4,152 (76.8%) | |
| Intermediate-risk group | 1,656 (25.7%) | 492 (48.0%) | 1,164 (21.5%) | |
| High-risk group | 205 (3.2%) | 112 (10.9%) | 93 (1.7%) | |
∗ Defined as blood pressure ≥140/90 mmHg or a self-reported history of hypertension and/or use of anti-hypertensive medication.
† Defined as total cholesterol ≥240 mg/dL or use of an anti-hyperlipidemic treatment.
‡ Coronary artery disease in a first-degree relative of any age.
| Characteristics | Overall (n=2,034) | Matched DM (n=1,017) | Matched non-DM (n=1,017) | Standardized Difference | P-value |
|---|---|---|---|---|---|
| Age (years) | 55.9±8.2 | 56.0±8.0 | 55.8±8.5 | 2.3% | 0.592 |
| Men | 1,725 (84.8%) | 852 (83.8%) | 873 (85.8%) | 5.8% | 0.182 |
| Body mass index (kg/m 2 ) | 25.5±3.0 | 25.3±3.0 | 25.6±3.0 | 7.0% | 0.100 |
| Waist circumference (cm) | 89.0±8.0 | 88.7±7.9 | 89.3±8.1 | 7.9% | 0.059 |
| Systolic blood pressure (mmHg) | 123.2±13.3 | 123.1±13.4 | 123.3±13.3 | 1.1% | 0.799 |
| Diastolic blood pressure (mmHg) | 78.2±10.3 | 78.1±10.1 | 78.3±10.4 | 2.0% | 0.651 |
| Hypertension ∗ | 1,074 (52.8%) | 528 (51.9%) | 546 (53.7%) | 1.3% | 0.421 |
| Hyperlipidemia † | 865 (42.5%) | 437 (43.0%) | 428 (42.1%) | 1.8% | 0.698 |
| Current smoker | 616 (30.3%) | 305 (30.0%) | 311 (30.6%) | 1.3% | 0.800 |
| Previous stroke | 29 (1.4%) | 15 (1.5%) | 14 (1.4%) | 0.8% | 0.999 |
| Family history of coronary artery disease ‡ | 258 (12.7%) | 130 (12.8%) | 128 (12.6%) | 0.6% | 0.946 |
| Total cholesterol (mg/dL) | 186.6±35.7 | 186.3±38.0 | 186.9±33.3 | 1.6% | 0.688 |
| Low-density lipoprotein cholesterol (mg/dL) | 113.6±30.9 | 113.1±32.7 | 114.2±28.9 | 3.5% | 0.379 |
| High-density lipoprotein cholesterol (mg/dL) | 50.5±12.4 | 50.6±12.2 | 50.3±12.6 | 2.6% | 0.540 |
| Triglyceride (mg/dL) | 151.6±96.3 | 153.4±98.0 | 149.8±94.6 | 3.7% | 0.399 |
| Creatinine (mg/dL) | 0.9±0.2 | 0.9±0.2 | 0.9±0.1 | 4.4% | 0.316 |
| Uric acid (mg/dL) | 5.6±1.3 | 5.6±1.3 | 5.6±1.3 | 2.6% | 0.564 |
| High-sensitivity C-reactive protein ≥2 mg/dL | 33 (1.6%) | 16 (1.6%) | 17 (1.7%) | 0.8% | 0.999 |
| Ejection fraction (%) | 63.4±4.1 | 63.4±4.2 | 63.4±3.9 | 0.5% | 0.919 |
∗ Defined as blood pressure ≥140/90 mmHg or a self-reported history of hypertension and/or use of anti-hypertensive medication.
† Defined as total cholesterol ≥240 mg/dL or use of an anti-hyperlipidemic treatment.
‡ Coronary artery disease in a first-degree relative of any age.
Table 3 lists the CCTA findings for matched subjects according to DM. Matched subjects with DM had higher CACS than those without DM. The incidence of any coronary atherosclerotic, calcified, or mixed plaque was significantly higher in matched subjects with DM. Matched subjects with DM also had higher plaque burden scores such as segment involvement score, segment stenosis score, and modified Duke prognostic score. Matched subjects with DM had higher more significant CAD in at least 1 coronary artery than those without DM (15.2% vs 10.2%, p = 0.001). However, these significant stenoses were mainly confined to 1-vessel coronary disease. There were no significant differences in significant CAD in the LM or proximal LAD, multivessel disease, or high-risk CAD between matched groups. During the follow-up period (median 21.8 months, interquartile range 15.2 to 33.4 months), a total of 58 cardiac events occurred in 56 patients: 8 all-cause deaths, 2 nonfatal MI, 1 acute coronary syndrome requiring hospitalization, and 47 coronary revascularizations. There was no significant difference in cardiac events between matched pairs (hazard ratio [HR] 1.438, 95% confidence interval [CI] 0.844 to 2.449, p = 0.181; Table 4 ).
| Coronary computed tomographic angiographic characteristics | Overall (n=2,034) | Matched DM (n=1,017) | Matched Non-DM (n=1,017) | P-value |
|---|---|---|---|---|
| Mean coronary artery calcium score | 76.1±212.2 | 89.9±240.4 | 62.8±179.5 | 0.004 |
| Any plaque | 1,115 (54.8%) | 594 (58.4%) | 521 (51.2%) | 0.001 |
| Plaque characteristics | ||||
| Calcified plaque | 798 (39.2%) | 430 (42.3%) | 368 (36.2%) | 0.004 |
| Non-calcified plaque | 473 (23.3%) | 255 (25.1%) | 218 (21.5%) | 0.057 |
| Mixed plaque | 274 (13.5%) | 166 (16.3%) | 108 (10.6%) | <0.001 |
| Segment involvement score | 1.6±2.1 | 1.7±2.2 | 1.4±2.1 | 0.001 |
| Segment stenosis score | 1.1±2.6 | 1.3±2.8 | 0.9±2.4 | 0.001 |
| Modified Duke prognostic score | 1.3±0.8 | 1.3±0.9 | 1.2±0.7 | 0.005 |
| Number of stenosed coronary arteries | ||||
| One vessel disease | 184 (9.0%) | 110 (10.8%) | 74 (7.3%) | 0.007 |
| Multi-vessel disease | 75 (3.7%) | 45 (4.4%) | 30 (2.9%) | 0.101 |
| Left main or proximal LAD artery | 93 (4.6%) | 54 (5.3%) | 39 (3.8%) | 0.138 |
| High-risk coronary artery disease ∗ | 13 (0.6%) | 44 (4.3%) | 27 (2.7%) | 0.058 |
∗ Defined as at least 2-vessel coronary disease with proximal left anterior descending artery involvement, 3-vessel disease, or left main disease.
| Clinical outcomes | Diabetes mellitus | Hazard ratio | 95% Confidence interval | P-value | |
|---|---|---|---|---|---|
| Yes | No | ||||
| Overall population (n=6,434) | |||||
| Death/ myocardial infarction /acute coronary syndrome | 6 (0.59%) | 19 (0.35%) | 1.669 | 0.667–4.180 | 0.274 |
| Death/ myocardial infarction /acute coronary syndrome /revascularization | 32 (3.12%) | 83 (1.53%) | 2.039 | 1.356–3.066 | 0.001 |
| Propensity-matched individuals (n=2,034) | |||||
| Death/ myocardial infarction /acute coronary syndrome | 7 (0.69%) | 4 (0.39%) | 1.757 | 0.514–6.003 | 0.368 |
| Death/ myocardial infarction /acute coronary syndrome /revascularization | 33 (3.24%) | 23 (2.26%) | 1.438 | 0.844–2.449 | 0.181 |
| Propensity-matched individuals at low-risk (n=838) | |||||
| Death/ myocardial infarction /acute coronary syndrome | 2 (0.48%) | 2 (0.48%) | 0.984 | 0.139–6.985 | 0.987 |
| Death/ myocardial infarction /acute coronary syndrome /revascularization | 9 (2.15%) | 3 (0.72%) | 3.009 | 0.815–11.113 | 0.098 |
| Propensity-matched individuals at intermediate-risk (n=600) | |||||
| Death/ myocardial infarction /acute coronary syndrome | 3 (1.00%) | 1 (0.33%) | 2.881 | 0.300–27.705 | 0.360 |
| Death/ myocardial infarction /acute coronary syndrome /revascularization | 16 (5.33%) | 5 (1.67%) | 3.197 | 1.171–8.730 | 0.023 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


