Patients with cardiovascular disease are increasingly exposed to low-dose ionizing radiation (LDIR) from diagnostic and therapeutic procedures. Previous studies have suggested that the malignancy risk associated with LDIR may be greatest in women and in young patients. We sought to compare the effect of LDIR on incident cancer across gender and age strata in a population-based cohort of patients with myocardial infarction (MI). All initially cancer-free patients with MI from 1996 to 2006 were identified in a province-wide administrative database. Procedure-specific LDIR dose estimates were used to generate a cumulative cardiac LDIR exposure variable. Time-dependent multivariate Cox regression was used to determine the relation between cardiac LDIR and incident cancer. A time-lag covariate of 3 years was used wherein a de novo cancer could only be attributed to LDIR incurred at least 3 years earlier. The effect of age and gender on LDIR-associated risk of cancer was evaluated with stratified models and the addition of interaction terms. The study cohort consisted of 56,606 men and 26,255 women. For each millisievert of cardiac LDIR, women were more likely to develop a cancer (hazard ratio 1.005, 95% confidence interval 1.002 to 1.008) than men (hazard ratio 1.002, 95% confidence interval 1.001 to 1.004) after adjusting for age, noncardiac LDIR, and covariates (p for interaction = 0.014). Contrarily, over the range studied (predominantly patients aged >50 years), age was not a determinant of LDIR-associated risk of cancer. In conclusion, women exposed to LDIR from cardiac imaging and therapeutic procedures after MI are at a greater risk of incident cancer compared with men after similar exposure. The extrapolated absolute risk from LDIR exposure would nonetheless be expected to be low.
Women have been found to have a greater risk than men of developing malignancy after similar exposure to low-dose ionizing radiation (LDIR), as have been younger patients. The risk in younger patients declines asymptotically with age, reaching a constant nadir at around the third decade of life. Using a large longitudinal population-based cohort, we previously suggested an association between LDIR exposure from medical cardiac imaging and therapeutic procedures after myocardial infarction (MI) and subsequent risk of malignancy. In the present study, we aimed to determine the risks posed to women and younger patients from LDIR exposure. We hypothesized, based on the findings from previous studies, that women would be at greater relative risk (RR) than men and that over the age range studied in our cohort (primarily >50 years of age), there would not be an interaction between age at exposure and cancer risk.
Methods
We previously described the creation of a cohort comprised of all patients with an MI in Quebec, Canada, from January 1, 1996 to March 31, 2006. Briefly, this was a population-based longitudinal cohort created by linking the Quebec hospital discharge summary database to provincial physicians’ services and drug claims databases as well as to vital status information. Linkage was done anonymously using patients’ unique, encrypted health-care insurance numbers. Patients with MI were identified by International Classification of Diseases-9 and -10 codes 410 and I21, respectively. This initial cohort contained 94,672 patients. We then excluded all patients with a concurrent or recent cancer diagnosis. Patients were excluded if, in the year preceding entry into the cohort or for 1 year thereafter, they had (1) an admission to hospital for a cancer diagnosis, (2) a cancer co-morbidity listed as a secondary diagnosis for a noncancer-related admission, (3) any outpatient visit with a cancer diagnosis, or (4) any visit billed by an oncologist, regardless of diagnosis. After exclusions, 82,861 patients remained. Figure 1 outlines the study design, which differed from the original design by incorporating a longer time-lag covariate, resulting in the exclusion of cancers previously included by the initial study design.
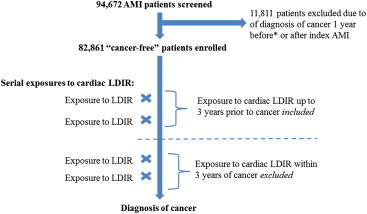
After index MI, exposure to 4 tests of interest was collected, and their respective LDIR doses estimated from published sources : myocardial perfusion imaging (15.6 mSv), diagnostic cardiac catheterization without intervention (7 mSv), cardiac catheterization with percutaneous coronary intervention (15 mSv), and cardiac resting ventriculography/multiple gated acquisition scan (7.8 mSv). The study did not incorporate gender-, body surface area-, or year-based LDIR dose estimates. Exposure to other common sources of medical LDIR was also collected for adjustment in multivariate analyses as some of these noncardiac tests could have been performed for work-up of cancer. Analyses were done with cumulative LDIR exposure expressed as continuous variables or in tertiles.
Follow-up began at the time of index MI. Cancer diagnoses were identified using International Classification of Diseases -9 and -10 codes. The criteria used for cancer diagnosis included (1) an admission for a cancer diagnosis, (2) a cancer co-morbidity listed as a secondary diagnosis for a noncancer-related admission, or (3) any outpatient visit for a cancer diagnosis.
Given that previous studies had shown a 5- to 10-year latency between LDIR exposure and cancer development, a 3-year time lag was subtracted from the time of cancer diagnosis to exclude all radiation within the 3 years preceding the cancer diagnosis ( Figure 1 ). As such, an incident cancer could only be attributed to LDIR exposure if the cancer occurred at least 3 years after the attributed radiation exposure. Three years was chosen to reflect the previously observed cancer latency time but was intentionally shorter to not exclude the possibility of earlier detection due to increased cancer risk. Because of this 3-year time-lag covariate, cancers with no associated LDIR exposure at least 3 years before diagnosis, including therefore those occurring within 3 years of MI, were not included. To explore whether longer time lags would affect our results, we performed sensitivity analyses using 5- and 7-year time-lag covariates.
To control for medical care access, we performed adjustments for rural patients (identified by Canadian postal codes with 0 in the second position) and for those who visited a general practitioner in the previous year. This was performed to control for “brought to medical attention” bias, wherein patients previously disconnected from the health-care infrastructure could be found to have a previously undiagnosed cancer at the time of presentation with MI. Furthermore, exclusion of any patient receiving a cancer diagnosis within 1 year of cohort entry would be expected to further minimize this potential bias.
Particularly radiosensitive cancers (thyroid, hematologic, bronchogenic, and breast) were also examined in isolation. Additionally, given that many of the imaging procedures studied irradiate a specific anatomic region, anatomically defined cancer groups were created: thorax/chest, abdomen, head-and-neck/central nervous system, bone/soft tissue, and hematologic. To determine site-specific hazard ratios (HRs), diagnosis of an initial cancer was not mutually exclusive of subsequent cancer diagnoses. For example, if a patient was diagnosed with colorectal cancer (abdominal group) and subsequently with non–small cell lung cancer (thorax/chest group), both cancers were used to determine their respective site-specific risks. For the overall determination of cancer risk, a patient was considered a “case” at the time of their first eligible cancer diagnosis.
Time-dependent Cox proportional hazards models were used to examine the association between LDIR exposure and cancer. For these adjusted risk analyses, only patients with qualifying cardiac LDIR exposure were included. We performed adjustment for potentially important variables, including age and gender, and also noncardiac LDIR and co-morbidities. After adjustment, to determine the extent to which residual confounding may have influenced our results, we determined the prostate cancer risk, which in the Life Span Study (LSS) was not related to LDIR exposure ; hence, any observed risk would serve as a “negative control” variable. The relation between continuous variables and the outcome was evaluated for linearity, and the proportional hazard assumption was tested for all variables (continuous and noncontinuous). All analyses were performed with SAS statistical software package (SAS Institute, Cary, North Carolina).
Results
The cohort consisted of 56,606 men (68%) and 26,255 women (32%). Women were generally older than men ( Table 1 ). Both female gender and advanced age were associated with a greater prevalence of co-morbid conditions such as hypertension, diabetes, congestive heart failure, chronic renal failure, and stroke.
| Characteristic | No Cardiac LDIR | First Tertile | Second Tertile | Third Tertile | ||||
|---|---|---|---|---|---|---|---|---|
| Women (%) | Men (%) | Women (%) | Men (%) | Women (%) | Men (%) | Women (%) | Men (%) | |
| Age (yrs) | 80.8 | 64.0 | 69.2 | 61.3 | 69.0 | 58.2 | 66.8 | 58.5 |
| Hypertension | 44 | 25 | 46 | 30 | 46 | 28 | 47 | 30 |
| Diabetes mellitus | 25 | 18 | 26 | 22 | 23 | 16 | 27 | 20 |
| Chronic heart failure | 28 | 15 | 20 | 15 | 14 | 10 | 18 | 13 |
| Stroke | 8 | 5 | 6 | 4 | 4 | 3 | 4 | 3 |
| Chronic renal failure | 12 | 8 | 6 | 5 | 5 | 4 | 6 | 4 |
| Shock | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| Treated by | ||||||||
| Cardiologist | 38 | 39 | 47 | 46 | 52 | 55 | 50 | 53 |
| Internist | 10 | 10 | 11 | 11 | 9 | 8 | 9 | 9 |
| GP | 52 | 50 | 42 | 43 | 39 | 38 | 40 | 38 |
| Visit to GP in the year before MI | 18 | 28 | 15 | 25 | 15 | 28 | 14 | 24 |
| Rural area | 24 | 30 | 20 | 25 | 23 | 26 | 24 | 26 |
| Noncardiac LDIR (mSv/yr) | 1.56 | 1.06 | 2.28 | 1.75 | 1.86 | 1.31 | 2.35 | 1.67 |
Women had a slightly less mean cardiac LDIR exposure in the first year after MI compared with men (14.6 ± 14.4 mSv for women vs 16.8 ± 14.4 mSv for men). Patients across the age strata received similar levels of radiation (1.09, 1.17, 1.15, and 0.99 mSv per 100 person-years for ages 45 to 54, 55 to 64, 65 to 74, and ≥75 years, respectively). The crude exposure stratified by age was 17.8, 18.6, 18.1, 16.5, and 10.5 mSv for ages <45, 45 to 54, 55 to 64, 65 to 74, and ≥75 years, respectively. Young men (<55 years) had the highest median cardiac LDIR exposure (15.0 mSv), whereas older women (≥75 years) had the lowest (7.0 mSv). The types of procedures were evenly distributed across gender strata, except percutaneous coronary intervention, which was performed consistently less frequently in women ( Figure 2 ).
During follow-up, a total of 12,020 cancers were diagnosed. Using the time-dependent model with the 3-year retrospective time lag that excluded cancers with no associated radiation exposure at least before 3 years, 6,934 new cancers were included in the analysis. The median time-to-cancer-diagnosis (of those cancers included in the analysis) after index MI was 5.6 years. The annual cancer incidence was similar in men and women (2.12 vs 1.96 per 100 person-years), whereas it progressively increased with advancing age (0.6, 1.25, 2.19, 3.08, and 2.72 per 100 person-years for ages <45, 45 to 54, 55 to 64, 65 to 74, ≥75 years, respectively). Variables independently associated with a cancer diagnosis, independent of LDIR exposure, included age, noncardiac LDIR, and access to care—as reflected by visits to a general practitioner during the past year ( Table 2 ).
| Variable | Women, HR (95% CI) | Men, HR (95% CI) |
|---|---|---|
| Cardiac LDIR (per mSv) | 1.005 (1.002–1.008) | 1.002 (1.001–1.004) |
| Age (per yr) | 1.024 (1.020–1.028) | 1.050 (1.047–1.052) |
| Hypertension | 1.037 (0.945–1.138) | 0.984 (0.923–1.049) |
| Diabetes | 1.045 (0.937–1.165) | 1.036 (0.962–1.115) |
| Heart failure | 0.962 (0.853–1.086) | 0.964 (0.883–1.052) |
| Arrhythmia | 1.106 (0.981–1.248) | 1.045 (0.967–1.130) |
| Chronic renal failure | 1.034 (0.815–1.313) | 1.059 (0.911–1.230) |
| Shock | 1.125 (0.723–1.751) | 1.363 (1.004–1.849) |
| Treated by cardiologist | 0.994 (0.901–1.096) | 1.005 (0.946–1.068) |
| Visit to GP in the year before MI | 1.013 (1.004–1.021) | 1.011 (1.004–1.018) |
| Rural area | 1.022 (0.918–1.138) | 0.974 (0.913–1.039) |
| Noncardiac LDIR (per mSv) | 1.008 (1.001–1.014) | 1.007 (1.002–1.011) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


