Although previous retrospective studies have suggested the clinical benefits of clopidogrel pretreatment in patients with ST-segment elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention (PPCI), the antiplatelet effect of thienopyridines during a narrow door-to-balloon time frame has not been evaluated. Seventy-nine consecutive patients with STEMI were treated with either 600 mg of clopidogrel (n = 49) or 60 mg of prasugrel (n = 30) loading on admission. All patients underwent PPCI with a door-to-balloon time of 48 ± 20 minutes. Adenosine diphosphate (ADP)–induced platelet aggregation (PA) was determined by light transmission aggregometry before thienopyridine loading, at PPCI, and after 72 hours. Baseline ADP-induced PA was comparable in clopidogrel- and prasugrel-treated patients (79 ± 10% vs 76 ± 9%, p = 0.2). Although ADP-induced PA was reduced significantly in both clopidogrel- and prasugrel-treated patients (p <0.01 for both), it was significantly lesser in prasugrel-treated patients (63 ± 18% vs 74 ± 12%, p = 0.002). Yet, <50% of the prasugrel-treated patients achieved adequate platelet inhibition (ADP-induced PA <70%) at PPCI. Prasugrel-treated patients, compared with clopidogrel-treated patients, were more likely to have Thrombolysis In Myocardial Infarction myocardial perfusion grade of ≥2 (79% vs 49%, p = 0.01), lower Thrombolysis In Myocardial Infarction frame count (10.2 ± 5.7 vs 13.6 ± 7.2, p = 0.03), and a numerically greater incidence of early ST-segment resolution >50% (26 of 30 [87%] vs 35 of 49 [71%], p = 0.1), suggesting better myocardial reperfusion. In conclusion, overall, prasugrel compared with clopidogrel pretreatment resulted in greater platelet inhibition at PPCI, but even with prasugrel, only <50% of the patients achieved early adequate platelet response.
Previous studies have shown that a clopidogrel loading dose given before nonurgent percutaneous coronary intervention results in better angiographic and clinical outcomes. The current American Heart Association/American College of Cardiology and European Society of Cardiology guidelines also advocate immediate administration of a clopidogrel or prasugrel loading dose in patients assigned to primary percutaneous coronary intervention (PPCI) for ST-segment elevation myocardial infarction (STEMI) as a class I recommendation. Indeed, a few retrospective observational studies have demonstrated lesser ischemic complications and/or better angiographic results with clopidogrel pretreatment in patients undergoing PPCI. However, the immediate antiplatelet effect of both clopidogrel and prasugrel has not been widely evaluated in this scenario. The aim of our study was to evaluate the immediate antiplatelet effect of thienopyridine pretreatment in patients undergoing PPCI for STEMI.
Methods
From August 2009 to January 2012, we prospectively evaluated 79 consecutive patients presenting with acute STEMI within 12 hours of symptom onset. Patients were evaluated and included in the study if they presented during regular working hours when platelet function testing was readily available. Diagnosis of STEMI was based on the presence of typical angina-type pain and ST elevation of ≥1 mm in ≥2 contiguous electrocardiographic leads. All patients were treated with a chewable 300-mg loading dose of aspirin. The first 49 patients received a 600-mg loading dose of clopidogrel on arrival at the emergency room or the intensive coronary care unit in the case of direct-transfer patients. After the approval of prasugrel by the Israeli Drug Administration, an additional 30 patients with STEMI eligible for prasugrel treatment (age <75 years, weight >60 kg, with no history of stroke) received an oral loading dose of 60 mg of prasugrel. Thereafter, patients received daily doses of 100 mg of aspirin and either 75 mg of clopidogrel or 10 mg of prasugrel. The decision of whether to use glycoprotein (GP) IIb/IIIa antagonists (tirofiban) at the time of PPCI was left to the discretion of the angiographer. Patients with cardiogenic shock, contraindication to thienopyridine treatment, renal failure (acute or chronic), or who were on warfarin therapy were excluded from the study.
Blood for platelet reactivity was drawn at 3 separate time points. The first sample was obtained immediately before thienopyridine loading, the second immediately before PPCI and GP IIb/IIIa antagonist administration, and the third 72 hours later. Blood was drawn with a loose tourniquet through a short venous catheter, collected into 3.2% sodium citrate–containing tubes, and was immediately assessed for platelet aggregation (PA). Blood samples were centrifuged and the upper fraction collected as platelet-rich plasma. The remaining blood was centrifuged again to obtain platelet-poor plasma. PA was evaluated by a turbidimetric PACKS-4 Aggregometer (Helena Laboratories, Beaumont, Texas) using adenosine diphosphate (ADP) 10 μM and arachidonic acid 1.6 mM as agonists. Changes in light transmission were recorded for 5 minutes and the maximal amplitude of aggregation was measured.
In accordance with contemporary practice and supported by previous studies, adequate response to thienopyridines was defined as achieving an ADP-induced PA of <70%. The sum of ST-segment elevation in all leads demonstrating ST-segment elevation was calculated from 2 electrocardiograms: on admission and immediately after PPCI. ST-segment elevation was measured 0.08 second after the J point. All measurements were performed by 2 cardiologists who were blinded to the angiographic findings and PA. A reduction of at least 50% in ST-segment elevation between the pre- and post-PPCI electrocardiograms was considered as significant ST-segment resolution (STR), and suggestive of adequate myocardial reperfusion.
Analysis of angiograms was performed after enrollment of the entire study cohort by 2 cardiologists blinded to the clinical findings and PA. Assessment of coronary blood flow was performed using the Thrombolysis In Myocardial Infarction (TIMI) grading system. Initial and final TIMI flow grades were recorded. Initial TIMI flow was defined as that first seen on the initial cine run and final TIMI flow as that seen on the last cine run after PPCI. Angiographic assessment of myocardial reperfusion was performed using the corrected TIMI frame count and TIMI myocardial perfusion grade (TMPG). A 2-dimensional echocardiographic study was obtained during hospitalization and evaluated for left ventricular ejection fraction and left ventricular wall motion index.
All patients were followed up throughout a 30-day period for development of clinical end points which included congestive heart failure, malignant arrhythmias (sustained ventricular tachycardia or ventricular fibrillation) of >48 hours after PPCI, target vessel revascularization, probable or definite stent thrombosis, reinfarction, stroke, and death. Patients were defined as having major adverse cardiovascular events if they sustained at least 1 of these adverse events throughout the 30-day follow-up period. The study was approved by the local research ethics board and all patients signed an informed consent form.
Normally distributed continuous variables are presented as mean ± SD. Unpaired and paired t tests were used for comparison of these continuous variables as indicated. Skewed continuous variables (time from symptom onset to admission and peak creatine phosphokinase) are presented as median and interquartile range and were compared using the Mann-Whitney test. Categorical variables are presented as percentages and were compared by chi-square or Fisher’s exact test as indicated. Ordinal variables (TIMI flow and TMPG) were compared using Kendall tau-b statistics and Fisher’s exact test in 2 × k cross tables. The relation between the time elapsed from thienopyridine loading dose to PPCI and PA at PPCI was studied separately in clopidogrel- and prasugrel-treated patients using linear regression of ADP-PA on time. The equality of slopes of the 2 study groups was estimated by the significance of the interaction between each of the 2 groups and the regression line of the entire study sample. To compare the consistency of distributions between clopidogrel- and prasugrel-treated patients, the Brown and Forsythe modification of the Levene’s test for comparison of variances was applied. No adjustments for multiple comparisons were used. All p values were 2 sided and those <0.05 were considered as significant. All calculations were performed using Stata 10 SE software (StataCorp LP, Texas).
Results
A total of 79 consecutive patients with STEMI were prospectively evaluated. All patients underwent PPCI with stenting. Forty-nine consecutive patients were treated with clopidogrel and 30 with prasugrel. The mean thienopyridine (door)-to-balloon time was 48 ± 20 minutes, with no significant differences between clopidogrel- and prasugrel-treated patients (49 ± 22 vs 46 ± 14 minutes, respectively, p = 0.3). As listed in Table 1 , patients treated with either prasugrel or clopidogrel had comparable baseline characteristics. The 2 study groups were also similar with respect to the use of GP IIb/IIIa antagonists (tirofiban) and aspiration devices at PPCI (63% vs 60%, p = 0.8% and 33% vs 30%, p = 0.9 for the clopidogrel and prasugrel groups, respectively).
| Variable | Clopidogrel, n = 49 (%) | Prasugrel, n = 30 (%) | p |
|---|---|---|---|
| Age (mean ± SD) (yrs) | 62 ± 13 | 60 ± 9.5 | 0.4 |
| Men | 41 (84) | 27 (90) | 0.4 |
| Smoker | 16 (33) | 13 (43) | 0.3 |
| Hypertension ∗ | 22 (45) | 11 (37) | 0.5 |
| Hyperlipidemia † | 22 (45) | 14 (47) | 0.9 |
| Diabetes mellitus | 9 (18) | 7 (23) | 0.9 |
| Body mass index (kg/m 2 ) (mean ± SD) | 26 ± 4 | 28 ± 4 | 0.1 |
| Time from symptom onset to admission (minutes) (median [IQR]) | 118 (85–180) | 118 (90–275) | 0.9 |
| Door-to-balloon time (minutes ± SD) | 49 ± 22 | 46 ± 14 | 0.3 |
| Previous use of medications | |||
| Aspirin | 16 (33) | 6 (27) | 0.3 |
| Statins | 15 (31) | 11 (38) | 0.5 |
| Proton pump inhibitors | 7 (15) | 2 (7) | 0.5 |
| ACE-I/ARBs | 9 (19) | 3 (10) | 0.5 |
∗ Hypertension was defined as either a known medical history of hypertension and/or currently treated for hypertension.
† Hyperlipidemia was defined as either a known medical history of hyperlipidemia and/or currently treated with low-density lipoprotein–lowering therapy.
Baseline ADP-induced PA before thienopyridine loading was comparable in clopidogrel- and prasugrel-treated patients (79 ± 10% vs 76 ± 9%, respectively, p = 0.2). Compared with preloading, ADP-induced PA was significantly reduced among both clopidogrel-treated (74 ± 12%, p <0.01) and prasugrel-treated patients (63 ± 18%, p <0.01) at PPCI. However, ADP-induced PA at PPCI was significantly less in patients treated with prasugrel compared with clopidogrel (63 ± 18% vs 74 ± 12%, p = 0.002) owing to a greater percent reduction in PA from preloading to PPCI (17 ± 21% vs 7.8 ± 8%, p = 0.04), despite a similar thienopyridine-to-balloon time ( Figure 1 ).
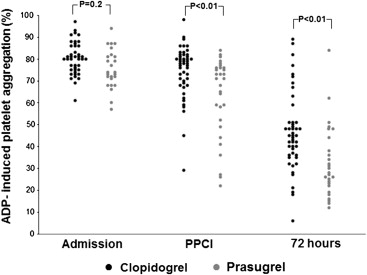
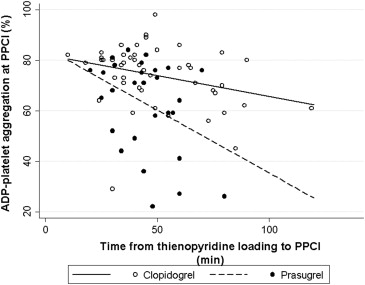
When time elapsed from thienopyridine loading dose to PPCI was correlated with PA at the time of PPCI, there was a significant, albeit moderate, negative relation for both clopidogrel-treated (r 2 = 0.1, p = 0.03) and prasugrel-treated patients (r 2 = 0.15, p = 0.04). As shown in Figure 2 , the regression lines, describing the relation between ADP-PA and the time that had elapsed from drug administration, were steeper for prasugrel than for clopidogrel (p <0.01), suggesting faster platelet inhibition with prasugrel. Yet, in both clopidogrel-treated (n = 14 [29%]) and prasugrel-treated patients (n = 14 [47%]), <50% achieved adequate platelet inhibition at the time of PPCI, defined as ADP-induced PA <70%, during a clinically relevant door-to-balloon time of about 50 minutes. Furthermore, although prasugrel was associated with a faster and more potent antiplatelet effect, this effect was not more consistent than that of clopidogrel (p by Brown-Forsythe test = 0.09).
At 72 hours after loading, patients treated with prasugrel, compared with those treated with clopidogrel, achieved markedly lesser ADP-induced PA (33 ± 16% vs 47 ± 18%, respectively, p = 0.0015). Accordingly, although only 1 prasugrel-treated patient (3%) was a nonresponder, 9 clopidogrel-treated patients (18%) were nonresponders.
Arachidonic acid–induced PAs on admission, at PPCI, and at 72 hours were 53 ± 25%, 36 ± 21%, and 21 ± 17%, respectively (p <0.01 vs baseline), with no significant differences between clopidogrel- and prasugrel-treated patients at all time points.
Consistent with its faster and more potent antiplatelet effect, prasugrel compared with clopidogrel therapy was associated with better angiographic indexes of microvascular reperfusion ( Table 2 ). Prasugrel-treated patients compared with clopidogrel-treated patients were more likely to have a TMPG of ≥2 (77% vs 49%, respectively, p = 0.01) and a lower TIMI frame count (10.2 ± 5.7 vs 13.6 ± 7.2, p = 0.03). Prasugrel loading was also associated with a numerically greater incidence of early STR (26 [87%] of 30 vs 35 [71%] of 49, p = 0.1). Despite similar ΣST elevation on the presenting electrocardiogram (7.8 ± 5.9 vs 7.1 ± 5.2, p = 0.4), suggestive of a similar area of jeopardized myocardium, prasugrel versus clopidogrel-treated patients had lower peak creatine phosphokinase (1,133 [600 to 2,280] vs 1,700 [945 to 3,427], p = 0.09) and higher left ventricular ejection fraction (48 ± 9 vs 43 ± 11, p = 0.02).
| Variable | Clopidogrel, n = 49 (%) | Prasugrel, n = 30 (%) | p |
|---|---|---|---|
| Baseline ADP platelet aggregation | 79 ± 10 | 76 ± 9 | 0.2 |
| ADP aggregation at PPCI | 74 ± 12 | 63 ± 18 | 0.002 |
| ADP aggregation at 72 hours | 47 ± 18 | 33 ± 16 | 0.0015 |
| Infarct-related artery: LAD | 21 (43) | 16 (53) | 0.4 |
| TIMI ≥2 before PPCI | 22 (45) | 18 (60) | 0.2 |
| TIMI 3 after PPCI | 41 (84) | 27 (90) | 0.6 |
| TMPG ≥2 | 24 (49) | 23 (77) | 0.01 |
| TIMI frame count (mean ± SD) | 13.6 ± 7.2 | 10.2 ± 5.7 | 0.03 |
| STR >50% | 35 (71) | 26 (87) | 0.12 |
| Ejection fraction before PPCI (%) (mean ± SD) | 43 ± 11 | 48 ± 9 | 0.02 |
| Regional wall index (mean ± SD) | 1.6 ± 0.36 | 1.5 ± 0.3 | 0.17 |
| Peak CPK (IU/L) (median [IQR]) | 1,700 (945–3,427) | 1,133 (600–2,280) | 0.09 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


