Anteroseptal ST elevation myocardial infarction (AS-STEMI), in which ST elevation is limited to leads V 1 to V 3 , is considered confined to the basal and mid anterior and septal segments, sparing the apex. In contrast, extensive anterior STEMI (EA-STEMI), in which ST elevation extends to leads V 4 to V 6 , is considered to involve more apical segments. However, it has been reported that AS-STEMI affects mainly the apex. Others have suggested that AS-STEMI may occur in patients with extensive anterior involvement if proximal occlusion of a wrapping left anterior descending coronary artery (LAD) results in cancelation of the basal-anterior and apical injury vectors. Therefore, the aim of this study was to identify, in 97 consecutive patients with STEMI, distinct coronary angiographic characteristics that could differentiate between cases of AS-STEMI (n = 39) and EA-STEMI (n = 58). Angiography was used to determine the length of the LAD, its site of occlusion, and whether there was an alternative blood supply to the apex. Patients with AS-STEMI were more likely than those with EA-STEMI to have ≥1 branches that reached the apex (p = 0.0015) and to have proximal LAD occlusion combined with either a short LAD or >1 large side branch (35.9% vs 12.1%, p = 0.011). However, patients with AS-STEMI were also more likely to have proximal occlusion before the first septal branch of a long LAD (35.9% vs 10.3%, p = 0.005). In conclusion, AS-STEMI can occur when only the basal and mid portions of the anterior wall are infarcted, but it can also arise when the infarction extensively involves the basal anterior and the distal inferior and apical segments.
Electrocardiography can be used to assess the size of the ischemic myocardium and the location of the infarct-related artery in acute ST elevation (STE) myocardial infarction (STEMI). In anterior STEMI (A-STEMI), electrocardiography is useful in determining the site of occlusion relative to the major side branches. A-STEMI has been classified as anteroseptal STEMI (AS-STEMI) when STE is limited to leads V 1 to V 3 and as extensive A-STEMI (EA-STEMI) when STE extends to leads V 4 to V 6 ( Figure 1 ). However, these anatomically based terms are probably misnomers, because neither angiographic nor echocardiographic data have correlated either injury pattern with the expected location of injury. Two opposing hypotheses have been offered to explain the occurrence of these 2 distinct electrocardiographic patterns of A-STEMI: (1) AS-STEMI represents A-STEMI in which the area of infarction is relatively small because there is an alternative blood supply to the distal segments (because of the presence of either a short left anterior descending coronary artery [LAD] and/or large diagonal or obtuse marginal branches), or (2) the AS-STEMI pattern occurs in patients with large areas of infarction because of the proximal occlusion of a long, wrapping LAD, resulting in deceptive attenuation of the expected STE in the lateral precordial leads. In this study, we analyzed data from patients with acute A-STEMI and compared electrocardiographic patterns with coronary angiographic findings to evaluate the influence of the site of occlusion, the morphology of the LAD, and the presence of large side coronary branches on the presenting electrocardiographic pattern.
Methods
We studied consecutive patients who were admitted with the diagnosis of first-time, acute A-STEMI as indicated by STE at the J point in ≥2 adjacent precordial leads (≥0.2 mV in leads V 2 and V 3 or ≥0.1 mV in the other precordial leads, V 1 or V 4 to V 6 ) and by other signs of myocardial ischemia, such as chest pain and elevated cardiac markers. All patients underwent urgent coronary angiography followed by either primary percutaneous coronary intervention or emergent coronary artery bypass grafting surgery at either the Michael E. DeBakey Veterans Affairs Medical Center or St. Luke’s Episcopal Hospital in Houston, Texas. The data from the Veterans Affairs hospital were obtained from a preexisting database of 172 consecutive patients who underwent primary percutaneous coronary intervention for STEMI from 2004 to 2008. The data from St. Luke’s Episcopal Hospital came from a preexisting database that lists all patients for whom the STEMI pager had been activated by the hospital’s emergency department or by hospital staff members from January 2007 to August 2009. The database contained data regarding 542 consecutive patients, of whom 305 underwent cardiac catheterization and 172 underwent acute reperfusion therapy. Index electrocardiograms from these hospitalizations were selected if they showed STE with positive T waves in ≥2 anterior leads (V 1 to V 6 ), typical evolution consistent with acute A-STEMI on follow-up electrocardiography (i.e., a decrease in STE magnitude with T-wave inversions after primary percutaneous coronary intervention), and elevated cardiac markers that confirmed myocardial necrosis.
Patients were excluded if their index electrocardiograms showed intraventricular conduction delay (left or right bundle branch block or nonspecific intraventricular conduction block), ventricular rhythms (including electronic ventricular pacing), or Wolff-Parkinson-White syndrome. Patients were also excluded if their electrocardiographic findings were consistent with partial reperfusion or an advanced stage of infarction (Q waves with negative T-waves), or if they had previously undergone coronary artery bypass grafting surgery.
Patients with A-STEMI were divided into 2 groups according to their electrocardiographic characteristics. An index electrocardiogram (obtained before revascularization) that showed STEs limited to leads V 1 to V 3 (STE ≥0.1 mV in lead V 1 and ≥0.2 mV in leads V 2 and V 3 ) was considered to indicate AS-STEMI, whereas an electrocardiogram with precordial STEs (≥0.1 mV) extending to lead V 4 , V 5 , or V 6 was considered to indicate EA-STEMI.
Of the 289 patients with acute STEMI from the 2 hospitals, 114 patients met the inclusion criteria and were selected for subsequent review of their coronary angiographic films. Films were primarily interpreted by 2 experienced angiographers who were blinded to each patient’s identity and group assignment (i.e., AS-STEMI or EA-STEMI). A third investigator adjudicated the review of the coronary angiographic films when needed. Angiographic characteristics of interest included the location of culprit occlusion within the LAD relative to the major diagonal and septal branches, the size of the LAD (short of the apex, reaches the apex, or wraps the apex), the presence or absence of ≥1 large side branch (≥2 mm in diameter; i.e., a large ramus intermedius, large obtuse marginal branch, or large diagonal branch proximal to site of culprit occlusion that reached the apical region of the left ventricle), initial and post–primary percutaneous coronary intervention Thrombolysis In Myocardial Infarction (TIMI) flow grade, the presence or absence of collaterals, and the number of diseased vessels (1, 2, or 3).
Discrete data are presented as absolute numbers and percentages. Continuous data are presented as mean ± SD. The chi-square test was used to analyze differences between discrete variables, and Student’s t test was used for continuous variables. A p value <0.05 was considered statistically significant.
Results
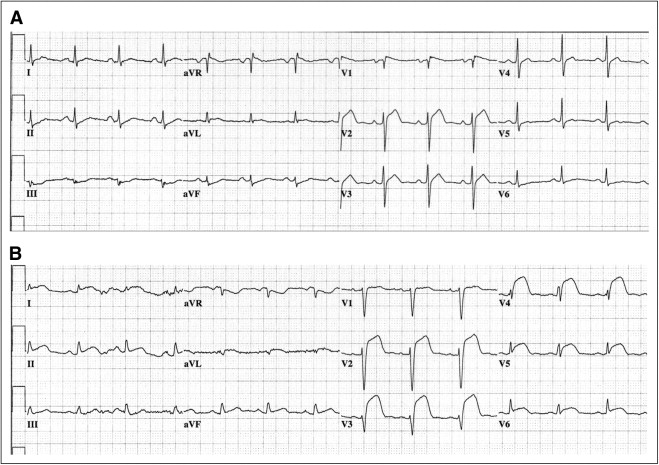
Of the 114 patients initially selected according to their electrocardiographic findings, 17 were excluded for various reasons: 8 patients underwent primary percutaneous coronary intervention on a vessel other than the LAD (5 right coronary artery, 1 left circumflex coronary artery, 1 with culprit lesions in the right and left circumflex coronary arteries, and 1 left main coronary artery), 4 patients did not have clear culprit lesions or significant coronary artery disease on coronary angiography, and 5 patients had missing angiographic data. Thus, 97 patients (12 women, 85 men; mean age 60.2 ± 12.1 years, range 29 to 90) met all criteria and were included in the study. Baseline and demographic data are listed in Table 1 . Patients with AS-STEMI tended to have lower peak creatine kinase levels and peak creatine kinase-MB levels than patients with EA-STEMI, but these differences did not reach statistical significance. The percentage of patients with peak cardiac troponin I levels <10 ng/ml was greater in the AS-STEMI group than in the EA-STEMI group. Figure 1 depicts sample electrocardiograms from patients with AS-STEMI and EA-STEMI.
| Variable | AS-STEMI (n = 39) | EA-STEMI (n = 58) | p Value |
|---|---|---|---|
| Men | 35 (90%) | 50 (86%) | 0.838 |
| Age (years) | 63.4 ± 12.0 | 58.3 ± 12.4 | 0.050 |
| Race | 0.453 | ||
| White | 22 (56%) | 35 (60%) | |
| Black | 11 (28%) | 15 (26%) | |
| Hispanic | 2 (5%) | 6 (10%) | |
| Other | 4 (10%) | 2 (3%) | |
| Coronary artery disease ⁎ , † | 16 (41%) | 23 (40%) | 0.939 |
| Myocardial infarction ⁎ | 5 (13%) | 11 (19%) | 0.603 |
| Percutaneous coronary intervention ⁎ | 12 (31%) | 11 (19%) | 0.273 |
| Diabetes mellitus ⁎ , ‡ | 11 (28%) | 13 (22%) | 0.683 |
| Hypertension ⁎ | 24 (62%) | 38 (66%) | 0.854 |
| Hyperlipidemia ⁎ , § | 22 (56%) | 30 (52%) | 0.806 |
| Heart failure ⁎ | 3 (8%) | 3 (5%) | 0.940 |
| Peak creatine kinase (U/L) | 1,223 ± 1816 | 1,794 ± 2114 | 0.168 |
| Peak creatine kinase-MB (ng/ml) | 103 ± 163 | 155 ± 226 | 0.193 |
| Peak troponin I (ng/ml) | 0.026 | ||
| <10 | 19 (49%) | 13 (22%) | |
| 10–50 | 10 (26%) | 22 (38%) | |
| >50 | 10 (26%) | 23 (40%) |
⁎ Established diagnosis in chart or receiving medications for this condition.
† History of myocardial infarction, effort-induced chest pain, or coronary angiography with documentation of coronary artery disease.
‡ Established diagnosis in chart, receiving medications for diabetes, or glycosylated hemoglobin ≥ 6.5%.
§ Established diagnosis in chart, receiving lipid-modifying medications, or abnormal lipid panel <24 hours after admission.
With regard to angiographic findings ( Table 2 ), patients with AS-STEMI were more likely to have proximal LAD occlusion than patients with EA-STEMI, but this difference was not statistically significant. There was no difference between the 2 groups in the size of the LAD (short, reaches the apex, or wraps around the apex) or in the number of vessels with >50% luminal diameter narrowing. Additionally, the prevalence of collaterals did not differ between the groups, and TIMI flow grades recorded before and after primary percutaneous coronary intervention were comparable between the groups.
| Variable | AS-STEMI (n = 39) | EA-STEMI (n = 58) | p Value |
|---|---|---|---|
| Site of LAD occlusion | |||
| Preseptal 1 | 16 (41%) | 15 (26%) | 0.178 |
| Prediagonal 1 | 18 (46%) | 19 (33%) | 0.263 |
| Prediagonal 1 and preseptal 1 | 14 (36%) | 12 (21%) | 0.154 |
| LAD size | 0.984 | ||
| Short | 3 (8%) | 4 (7%) | — |
| Reaches apex | 9 (23%) | 14 (24%) | — |
| Wraps the apex | 27 (69%) | 40 (69%) | — |
| Side branches that supply the apex | |||
| Large diagonal | 11 (28%) | 8 (14%) | 0.136 |
| Large ramus intermedius | 6 (15%) | 6 (10%) | 0.671 |
| Large obtuse marginal | 33 (85%) | 28 (48%) | 0.0006 |
| Number branches that supply the apex | 0.0015 | ||
| 0 | 5 (13%) | 27 (47%) | — |
| 1 | 21 (54%) | 24 (41%) | — |
| 2 | 12 (31%) | 5 (9%) | — |
| 3 | 1 (3%) | 2 (3%) | — |
| Number of narrowed coronary arteries | 0.351 | ||
| 1 | 10 (26%) | 20 (35%) | — |
| ≥2 | 29 (74%) | 38 (66%) | — |
| Collaterals | 24 (62%) | 42 (72%) | 0.366 |
| TIMI flow before primary percutaneous coronary intervention | 0.904 | ||
| 0 | 27 (69%) | 37 (64%) | |
| 1 | 4 (10%) | 7 (12%) | |
| 2 | 6 (15%) | 9 (16%) | |
| 3 | 2 (5%) | 5 (9%) | |
| TIMI flow after primary percutaneous coronary intervention | 0.084 | ||
| 0 | 2 (5%) | 5 (9%) | |
| 1 | 1 (3%) | 4 (7%) | |
| 2 | 7 (18%) | 2 (3%) | |
| 3 | 29 (74%) | 47 (81%) |
Patients with AS-STEMI more often had large obtuse marginal branches that reached the apex, but the prevalence of large diagonal branches or a large ramus intermedius did not significantly differ between the AS-STEMI and EA-STEMI groups. Overall, significantly more patients with AS-STEMI had ≥1 branch that supplied the apex ( Table 2 ).
When we examined potential predictors of ischemia extent ( Table 3 ), we found that proximal occlusion of a short LAD before the first septal branch was relatively rare in our cohort. Compared with the patients with EA-STEMI, more of the patients with AS-STEMI had coronary anatomy that favored ischemia that was limited to the basal and mid segments of the left ventricle and that spared the apex. Patients in the AS-STEMI group were 3 times as likely as patients in the EA-STEMI group to have a proximal occlusion of the LAD before the first septal branch combined with either a short LAD that did not reach the apex or ≥1 large side branch that supplied the apex (p = 0.011).



