Few studies have investigated whether angiotensin II receptor blocker (ARB) is a practical alternative to angiotensin-converting enzyme inhibitor (ACEI) for long-term use after acute myocardial infarction (AMI) in real-world practice in the percutaneous coronary intervention era. We compared 5-year survival benefits of ACEI and ARB in patients with AMI registered in the Osaka Acute Coronary Insufficiency Study. Study subjects were divided into 3 groups: ACEI (n = 4,425), ARB (n = 2,158), or patients without either drug (n = 2,442). A total of 661 deaths were recorded. Cox regression analysis revealed that treatment with either ACEI or ARB was associated with reduced 5-year mortality (adjusted hazard ratio [HR] 0.70, 95% confidence interval [CI] 0.58 to 0.83, p <0.001 and HR 0.79, 95% CI 0.64 to 0.98, p = 0.03, respectively). However, Kaplan-Meier estimates and Cox regression analyses based on propensity score revealed that ACEI was associated with better survival than ARB from 2 to 5 years after survival discharge (adjusted HR 0.53, 95% CI 0.38 to 0.74, p <0.001). These findings were confirmed in a propensity score–matched population. In conclusion, treatment with ACEI was associated with better 5-year survival after AMI.
Angiotensin-converting enzyme inhibitor (ACEI) was the first clinically approved renin-angiotensin-aldosterone system (RAS) inhibitor, and much evidence presented in the 1990s and early 2000s have demonstrated the effectiveness of ACEI for improving cardiovascular disease–related morbidity and mortality. Angiotensin II receptor blocker (ARB) has also been examined clinically for cardiovascular disease treatment. Based on the results of 2 randomized clinical trials (RCTs) such as Optimal Trial in Myocardial Infarction with Angiotensin II Antagonist Losartan (OPTIMAAL) and the Valsartan in Acute Myocardial Infarction (VALIANT), which examined clinical impacts of ARB after acute myocardial infarction (AMI), the international guidelines recommend that ACEI should be used as the first-line treatment after AMI and that ARB should be considered in patients who are intolerant to ACEI therapy. We investigated whether ACEI and ARB had comparable long-term benefits in a large cohort of post-AMI patients registered in the Osaka Acute Coronary Insufficiency Study (OACIS).
Methods
The OACIS is a prospective, multicenter, observational study enrolling consecutive patients with AMI at 25 collaborating hospitals in the Osaka region of Japan. The OACIS is registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR) in Japan (ID: UMIN000004575). Details of OACIS are described elsewhere ( Supplementary Material ).
The diagnosis of AMI was based on the World Health Organization criteria, which required 2 of the following 3 criteria to be met: (1) clinical history of central chest pressure, pain, or tightness lasting ≥30 minutes; (2) ST-segment elevation >0.1 mV in at least 1 standard or 2 precordial leads; and (3) an increase in serum creatine phosphokinase concentration of more than twice the normal laboratory value. Research cardiologists and trained research nurses recorded data concerning sociodemographic variables, medical history, therapeutic procedures, and clinical events during the patient’s hospital stay. The present study protocol complied with the Declaration of Helsinki and was approved by the institutional ethical committee of each participating institution. All study candidates were informed about data collection and blood sampling, and written informed consents were obtained.
A flowchart of patient selection is presented in Supplementary Figure 1 . A total of 4,425 patients treated with ACEI at discharge, 2,158 with ARB, and 2,442 prescribed neither ACEI nor ARB (no RAS inhibitor) were enrolled in the present study. A direct comparison of survival benefit was performed between patients treated with ACEI and those with ARB at discharge. For the inverse probability of treatment weighting (IPTW) method using propensity score (PS), 5,563 eligible patients without missing data for Cox regression analysis were selected (3,784 and 1,779 patients with ACEI or ARB at discharge, respectively). For PS-matched analysis, 3,268 patients (1,634 in each treatment group) were selected and analyzed.
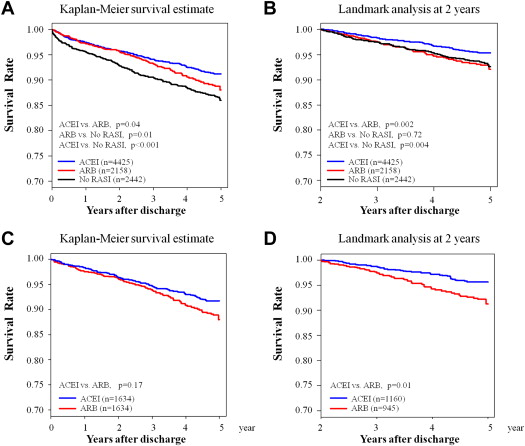
The primary end point was all-cause death, and the secondary end points were heart failure hospitalization and nonfatal re-myocardial infarction. For patients discharged alive, follow-up clinical data were obtained for 5 years. Categorical variables were compared by chi-square tests, and continuous variables were compared by the Kruskal-Wallis test for 3-group comparison (ACEI, ARB, and no RAS inhibitor) and Wilcoxon rank sum test for 2-group comparison (ACEI and ARB). The annual trend in the prescription rate of ACEI or ARB was assessed by the Cochran-Armitage trend test ( Supplementary Figure 2 ). The Kaplan-Meier method was used to estimate event rates, and the differences were assessed by the log-rank test. Landmark analysis of the primary end point was also performed 2 years after survival discharge. Specifically, survival estimates were calculated in patients without any adverse events 2 years after survival discharge, because the Kaplan-Meier survival estimates for the ACEI and ARB treatment groups appeared to differentiate at this time point ( Figure 1 ).
Inter- and intra-class drug differences in survival benefit were compared by age and sex-adjusted Cox regression analyses, and the hazard ratio (HR) and 95% confidence intervals (CI) were calculated using data obtained from the 2,442 patients without RAS inhibitors as a reference ( Supplementary Figure 1 ). To reduce potential confounding effects due to patient background variability in the direct comparison between ACEI and ARB, the PS method was used in combination with Cox regression modeling. PS was defined as the probability of treatment assignment conditional on the measured baseline covariates. The inverse probability of treatment weighting method based on the PS was used to reduce confounding in time-to-event observational data. To confirm the robustness of the inverse probability of treatment weighting results, we also performed PS matching with a caliper width of 0.001. For the estimation of PS, we used a logistic regression model in which the treatment status (ACEI or ARB) was regressed on the following baseline characteristics: age, gender, body mass index, diabetes, hypertension, dyslipidemia, smoking, previous myocardial infarction, ST elevation myocardial infarction, Killip’s classification, reperfusion therapy, and prescription of β blockers, calcium channel blockers, statins, diuretics, and antiplatelet agents. Statistical significance was set as p <0.05. All statistical analyses were performed using SAS, version 9.3 (SAS Institute Inc., Cary, North Carolina), or R software packages, version 2.15.1 (R Development Core Team, Vienna, Austria).
The corresponding author had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis.
Results
Patient characteristics based on treatment group are summarized in Table 1 . Significant differences in nearly all background variables were detected among the ACEI, ARB, and no RAS inhibitor treatment groups. Notably, patients in the no RAS inhibitor group were less frequently treated with evidence-based medications. Between the ACEI and ARB treatment groups, patients who received ACEI had lower prescription rates for state-of-the-art medications at discharge, such as β blockers and statins, partly because these patients were likely registered in the earlier period of the OACIS registry ( Supplementary Figure 2 ). In the PS-matched cohort, patient characteristics were well balanced ( Supplementary Table 1 ).
| Parameter | No RASI (n = 2442) | ACEI (n = 4425) | ARB (n = 2158) | p-Value (Total) | p-Value (ACEI vs ARB) |
|---|---|---|---|---|---|
| Age (years) | 67 (59–75) | 65 (57–73) | 67 (59–75) | <0.001 | <0.001 |
| Men | 73.6% | 77.9% | 74.3% | <0.001 | 0.001 |
| Body mass index (kg/m 2 ) | 23.0 (21.0–25.2) | 23.5 (21.5–25.7) | 23.9 (21.6–26.0) | <0.001 | 0.001 |
| ST-elevation myocardial infarction | 82.3% | 86.8% | 83.7% | <0.001 | <0.001 |
| Diabetes mellitus | 34.7% | 32.6% | 34.0% | 0.19 | 0.27 |
| Hypertension | 49.4% | 59.3% | 70.3% | <0.001 | <0.001 |
| Dyslipidemia | 40.6% | 44.8% | 46.5% | <0.001 | 0.19 |
| Smoking | 59.3% | 66.0% | 61.5% | <0.001 | <0.001 |
| Previous myocardial infarction | 13.6% | 11.9% | 10.8% | 0.02 | 0.18 |
| KILLIP class | <0.001 | 0.01 | |||
| 1 | 79.5% | 85.4% | 84.2% | ||
| 2 | 9.1% | 8.4% | 7.4% | ||
| 3 | 4.1% | 3.3% | 4.4% | ||
| 4 | 7.3% | 2.9% | 4.0% | ||
| Emergent coronary angiography | 92.7% | 95.3% | 96.2% | <0.001 | 0.10 |
| Target Lesion | <0.001 | 0.22 | |||
| Left main | 3.1% | 0.9% | 1.3% | ||
| Left anterior descending artery | 38.6% | 47.9% | 46.2% | ||
| Right coronary artery | 38.7% | 34.9% | 34.2% | ||
| Left circumflex artery | 16.3% | 12.9% | 14.8% | ||
| Diagonal branch | 3.0% | 3.2% | 3.4% | ||
| Graft | 0.4% | 0.1% | 0.1% | ||
| Reperfusion therapy | |||||
| Percutaneous coronary intervention | 80.4% | 89.8% | 93.2% | <0.001 | <0.001 |
| Thrombolysis | 8.2% | 7.1% | 6.6% | 0.12 | 0.49 |
| Coronary artery bypass graft | 6.6% | 0.9% | 1.4% | <0.001 | 0.07 |
| Hemoglobin A1c (%) | 6.0 (5.56.9) | 5.9 (5.5–6.9) | 6.0 (5.6–7.0) | 0.01 | 0.001 |
| Total cholesterol (mg/dL) | 187 (158–218) | 190 (164–220) | 193 (166–224) | <0.001 | 0.02 |
| Low-density lipoprotein cholesterol (mg/dL) | 113 (87–139) | 122 (99–148) | 124 (101–149) | <0.001 | 0.57 |
| High-density lipoprotein cholesterol (mg/dL) | 45 (37–53) | 44 (38–53) | 44 (37–52) | 0.78 | 0.49 |
| Triglyceride (mg/dL) | 91 (60–137) | 94 (60–143) | 99 (64–149) | <0.001 | 0.002 |
| Estimated glomerular filtration rate (mL/min/1.73 m 2 ) | 47.9 (33.8–61.8) | 51.8 (41.2–64.5) | 52.8 (40.9–65.4) | <0.001 | 0.35 |
| Peak creatine phosphokinase (IU/L) | 1701 (793–3400) | 2025 (925–3801) | 1793 (910–3503) | <0.001 | 0.02 |
| Echocardiography data | |||||
| Left ventricular end-diastolic dimension (mm) | 50.0 (46.0–54.0) | 50.0 (46.5–54.0) | 50.9 (47.0–55.0) | 0.04 | 0.03 |
| Left ventricular end-systolic dimension (mm) | 34.0 (30.0–40.0) | 34.0 (30.0–39.0) | 34.0 (30.0–39.0) | 0.051 | 0.04 |
| Left ventricular ejection fraction (%) | 52.6 (43.8–60.3) | 53.5 (44.6–60.7) | 55.6 (46.3–62.2) | <0.001 | <0.001 |
| Medication at discharge | |||||
| Beta-blocker | 35.5% | 48.8% | 62.9% | <0.001 | <0.001 |
| Calcium channel blocker | 26.0% | 18.6% | 19.7% | <0.001 | 0.27 |
| Statin | 29.7% | 39.6% | 57.0% | <0.001 | <0.001 |
| Antiplatelet | 91.0% | 98.1% | 98.5% | <0.001 | 0.35 |
| Diuretic | 30.7% | 26.9% | 26.5% | <0.001 | 0.74 |
| Follow-up duration (days) | 1416 (345–1792) | 1635 (707–1798) | 1032 (343–1737) | <0.001 | <0.001 |
Annual trends in the prescription rate of RAS inhibitors are shown in Supplementary Figure 2 . The prescription rate of ARB had increased annually until 2007, whereas that of ACEI decreased. In 2010, approximately 80% of all study patients received RAS inhibitors at discharge. The types of ACEI and ARB prescribed at discharge are listed in Supplementary Table 2 .
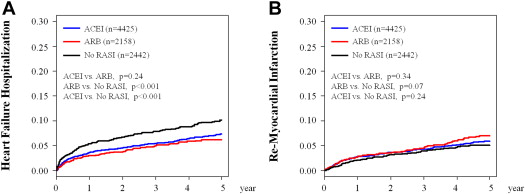
A total of 661 deaths (no RAS inhibitor, 231; ACEI, 293; and ARB, 137), 512 heart failure hospitalizations (no RAS inhibitor, 174; ACEI, 250; and ARB, 88), and 375 nonfatal re-myocardial infarctions (no RAS inhibitor, 85; ACEI, 200; and ARB, 90) were recorded during a median follow-up period of 3.9 years (median 1,426 days, interquartile range 402 to 1,794). Kaplan-Meier survival analysis demonstrated that both the ACEI and ARB groups had better 5-year mortality than the no RAS inhibitor group ( Figure 1 ). Age and sex-adjusted Cox regression analysis revealed that both ACEI and ARB treatments were associated with reduced 5-year mortality compared with no RAS inhibitor treatment (adjusted HR 0.70, 95% CI 0.58 to 0.83, p <0.001 for ACEI and adjusted HR 0.79, 95% CI 0.64 to 0.98, p = 0.03 for ARB, respectively). However, treatment with ACEI was associated with significantly lower 5-year mortality compared with that with ARB ( Figure 1 ). Landmark analysis demonstrated that the superiority of ACEI with regard to long-term prognostic impact was only evident after 2 years of discharge. In addition, the survival estimate of the ARB group from 2 to 5 years after survival discharge was comparable to that of the no RAS inhibitor group ( Figure 1 ). These observations were consistent with those obtained in the PS-matched cohort ( Figure 1 ). In contrast to the survival rates, no significant differences in heart failure hospitalization or nonfatal re-myocardial infarction rates were detected between the ACEI and ARB groups ( Figure 2 ).