Race has seldomly been reported in the major clinical trials of cardiac resynchronization therapy (CRT). When described, African Americans (AAs) were substantially under-represented. This study sought to compare reverse ventricular remodeling and long-term outcomes in AAs versus European Americans (EAs) with advanced heart failure who underwent CRT. We extracted demographic (including race), clinical, and echocardiographic data on patients with advanced heart failure who underwent CRT with a left ventricular ejection fraction (LVEF) ≤35% and a QRS duration ≥120 ms. Long-term outcomes were compared between AAs and EAs. In patients in whom follow-up echocardiograms were available, improvement in LVEF (defined as an absolute improvement ≥5%) was compared between races. From a cohort of 662 patients, there were 88 AAs and 574 EAs. At a mean follow-up of 5.0 ± 2.5 years, survival rate free of left ventricular assist device (LVAD) and heart transplant was 54.5% for AAs and 53.8% for EAs (log-rank p = 0.997). In multivariate analysis, there was no difference in survival free of heart transplant or LVAD based on race (hazard ratio 1.1 [0.74 to 1.56], p = 0.72, EAs race as referent); 424 patients had a follow-up echocardiogram (55.4% EAs and 64.7% AAs). In multivariate analysis, there was no difference in the incidence of response based on race (1.1 [0.6 to 2.1, p = 0.80], EAs as referent). AAs derive similar benefits with CRT compared with EAs in terms of improvement in LVEF and long-term survival free of LVAD and heart transplant.
African Americans (AAs) are less likely to receive guideline-based therapy compared with European Americans (EAs) despite their high burden of cardiovascular disease. This applies also to utilization of cardiac resynchronization therapy (CRT), although the disparity may be improving. Even large-scale registries, for example Evidence-Based Heart Failure Therapies in the Outpatient Setting (IMPROVE-HF), have included relatively small numbers of AAs. Randomized controlled trials of CRT have generally not reported racial characteristics of enrolled patients. When reported, AAs were significantly under-represented. This omission is significant because AAs are a vulnerable subgroup. Several studies have suggested that AAs have impaired outcomes with multiple cardiovascular disease states including ischemic heart disease and sudden cardiac death compared with EAs. Notably, poorer outcomes persist even after guideline-based treatment. Reasons are uncertain but likely involve a complex interplay of socioeconomic factors, co-morbidities, and genetics. Whether these conditions affect therapy with implantable cardiac electronic devices, however, is unclear. The few studies that have assessed the interaction of race and outcomes after defibrillator or CRT implant have been limited by small numbers, relatively short follow-up periods, only a few types of end points reported and, for CRT, have contained mostly minimally symptomatic patients. Some results suggest lesser benefit in AAs, questioning the utility of implantable device therapy altogether. In the present study, we sought to determine the incidence of reverse ventricular remodeling and long-term survival free of left ventricular assist device (LVAD) or heart transplant in AA CRT candidates with advanced HF (New York Heart Association [NYHA] class III/IV) compared with an EA control.
Methods
This retrospective study involved the analysis of a consecutive cohort of patients who underwent the new implantation of a CRT device at the Cleveland Clinic, Cleveland, Ohio, from September 30, 2003, to August 6, 2007. The study was approved by the institutional review board of the Cleveland Clinic for retrospective medical records review and performed according to institutional guidelines. Clinical, electrocardiographic, and echocardiographic data were gathered through chart review. For inclusion in the final cohort, all patients had a left ventricular ejection fraction (LVEF) ≤35%, a QRSd ≥120 ms, and documentation of race in the medical record. Racial status was determined through review of the demographic profile in the patient’s medical record. Such data are originally entered into the medical record from questionnaire data the patient had filled out. An assessment of mortality was made using the US Social Security Death Index searched in August 2012. The subsequent implantation of an LVAD or heart transplant was assessed using current Cleveland Clinic advanced heart failure (HF) therapy registry data. Kaplan-Meier curves were constructed, and a multivariate model was created to compare outcomes between AAs and Caucasians accounting for many possible confounders. In patients in whom follow-up echocardiograms were available, an assessment of reverse remodeling was made. Response was defined as an absolute improvement in LVEF of ≥5%.
In the cohort as a whole, CRT device implantations were performed transvenously in the vast majority of patients by electrophysiologists targeting a lateral or posterolateral vein for the left ventricular lead position. In instances when a transvenous lead could not be placed because of procedural difficulty, a minimally invasive epicardial lead through a mini-thoracotomy was placed by a staff cardiothoracic surgeon.
Continuous variables were presented as a mean ± SD and dichotomous variables as an absolute number with percentage. Comparisons between continuous variables were made using the Student’s t test for parametric variables and a Mann-Whitney test for nonparametric variables. Dichotomous variables were compared using Fisher’s exact test. Kaplan-Meier curves using the log-rank test were created to assess survival free of LVAD or heart transplant over the duration of follow-up. A multivariate Cox proportional hazards model was created to compare survival free of LVAD or heart transplant in ASs compared with EAs controlling for multiple variables selected based on a priori knowledge. To test the Cox assumption that the hazard ratio between subjects is constant, a time-varying covariate was entered into the model for each variable with a p value >0.05 needed to satisfy this assumption. In those patients in whom a follow-up echocardiogram was available, multivariate logistic regression analysis was performed to determine the association between racial status and response controlling for multiple a priori determined variables. All analyses were performed using SPSS Inc. (Chicago, Illinois).
Results
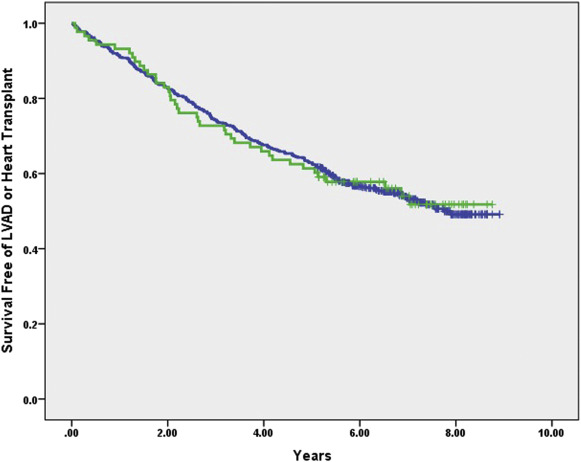
From September 30, 2003, to August 6, 2007, 662 patients met inclusion criteria (88 AAs and 574 EAs). The baseline characteristics of the cohort are listed in Table 1 . Given the time period from whence this cohort was derived, patients had class III or IV HF. Compared with EAs, AAs in this cohort were younger, more commonly women, had a lower LVEF and greater left ventricular dilation, were more commonly nonischemic, and had a higher incidence of hypertension and anemia. Over a mean follow-up of 5.0 ± 2.5 years, there were 305 end points (14 LVADs, 14 heart transplants, and 277 deaths). In univariate analysis, there was no difference in outcomes between AAs and EAs over the duration of follow-up (log-rank p = 1.0; Figure 1 ). In multivariate analysis controlling for age, history of atrial fibrillation, cardiomyopathy subtype, QRS duration, left bundle branch block, creatinine, concomitant ICD capability (CRT-defibrillators vs p), baseline ejection fraction, β-blocker use, ace inhibitor use, and antiarrhythmic drug use, there was no difference in outcomes in AAs (hazard ratio 1.1 [0.74 to 1.56], p = 0.72) compared with EAs (as referent; Table 2 ).
| Variable | Total (n=662) | African American (n=88) | European Americans (n=574) | p-value |
|---|---|---|---|---|
| Age (years) | 66.7±11.7 | 63.1±11.4 | 67.2±11.7 | 0.002 |
| Men | 466(70%) | 47(53%) | 419(73%) | <0.001 |
| Baseline left ventricular ejection fraction | 21.2±7.0 | 18.6±6.5 | 21.5±7.0 | <0.001 |
| Baseline left ventricular end diastolic diameter(cm) | 6.2±1.0 | 6.4±1.3 | 6.1±1.0 | 0.12 |
| Baseline left ventricular end systolic diameter(cm) | 5.2±1.2 | 5.5±1.3 | 5.1±1.1 | 0.01 |
| Ischemic cardiomyopathy | 394(60%) | 38(43%) | 356(62%) | 0.001 |
| Cardiac resynchronization therapy device with defibrillator | 638(96%) | 85(97%) | 553(96%) | 1.0 |
| Serum hemoglobin (g/dl) | 12.6±2.0 | 12.2±1.8 | 12.7±2.0 | 0.02 |
| Serum creatinine (mg/dl) | 1.4±0.7 | 1.5±1.0 | 1.3±0.7 | 0.14 |
| QRS duration(ms) | 165.4±26.4 | 161.1±22.2 | 166.1±27.0 | 0.06 |
| Left bundle branch block | 282(43%) | 45(51%) | 237(41%) | 0.08 |
| History of atrial fibrillation (any type) | 352(53%) | 39(44%) | 313(55%) | 0.09 |
| Chronic obstructive pulmonary disease | 95(14%) | 12(14%) | 83(15%) | 1.0 |
| Hypertension | 396(60%) | 63(72%) | 333(58%) | 0.02 |
| Hyperlipidemia | 376(57%) | 50(57%) | 326(57%) | 1.0 |
| Prior malignancy | 87(13%) | 15(17.0%) | 72(13%) | 0.24 |
| Diabetes mellitus | 248(38%) | 41(47%) | 207(36%) | 0.08 |
| Prior stroke or transient ischemic attack | 84(13%) | 14(16%) | 70(12%) | 0.39 |
| Beta-adrenergic blocker | 525(79%) | 75(85%) | 450(78%) | 0.28 |
| Angiotensin converting enzyme inhibitor or angiotensin II receptor blocker | 509(77%) | 72(82%) | 437(76%) | 0.47 |
| Diuretic | 513(78%) | 74(84%) | 439(77%) | 0.24 |
| Anti-arrhythmic medications | 138(21%) | 16(18%) | 122(21%) | |
| Nitrates | 188(28%) | 25(28%) | 163(28%) | 1.0 |
| Hydralazine | 67(10%) | 17(19%) | 50(9%) | 0.007 |
| Clopidogrel | 98(15%) | 10(11%) | 88(15%) | 0.34 |
| Digoxin | 292(44%) | 35(40%) | 257(45%) | 0.30 |
| Variable | Hazard Ratio | p-value |
|---|---|---|
| African American Race (European American race as referent) | 0.98(0.67-1.43) | 0.93 |
| Age | 1.02(1.008-1.03) | 0.002 |
| Male gender | 1.49(1.06-2.10) | 0.02 |
| Baseline left ventricular ejection fraction | 0.97(0.95-0.98) | <0.001 |
| History of atrial fibrillation | 1.26(0.98-1.63) | 0.07 |
| Ischemic cardiomyopathy | 1.29(0.97-1.71) | 0.08 |
| Cardiac resynchronization therapy device with defibrillator | 1.39(0.64-2.99) | 0.41 |
| Serum creatinine (mg/dl) | 1.51(1.32-1.73) | <0.001 |
| QRS duration(ms) | 0.989(0.984-0.994) | <0.001 |
| Left bundle branch block | 0.74(0.56-0.96) | 0.02 |
| Beta blocker use | 0.85(0.62-1.15) | 0.29 |
| Angiotensin converting enzyme inhibitor or angiotensin II receptor blocker | 0.76(0.57-1.004) | 0.05 |
| Anti-arrhythmic drug use | 1.43(1.09-1.89) | 0.01 |
A follow-up echocardiogram was available in 424 patients: 57 in AAs and 367 in EAs. The follow-up echocardiogram was performed at a median of 9.2 months (25% to 75%, interquartile range 5.5% to 14.2%) after CRT implant. From this cohort, 65.1% met criteria for response (66.7% of AAs and 64.9% of Caucasians, p = 0.88; Table 3 ). In multivariate analysis controlling for gender, baseline LVEF, baseline left ventricular end-diastolic diameter, a history of atrial fibrillation, cardiomyopathy subtype, QRS duration, and QRS morphology, race was not a significant predictor of response (odds ratio 0.81 [0.41 to 1.57], p = 0.53; Table 4 ; Caucasian race as referent).
| Variable | Total (n=424) | Responders (n=276) | Non-responders (n=148) | p-value |
|---|---|---|---|---|
| Age (years) | 66.2±11.9 | 66.4±12.0 | 65.8±11.8 | 0.59 |
| Men | 294(69%) | 175(63%) | 119(80%) | <0.001 |
| African Americans | 57(13%) | 38(14%) | 19(13%) | 0.882 |
| Baseline left ventricular ejection fraction | 21.2±7.1 | 20.7±7.1 | 22.1±7.0 | 0.04 |
| Baseline left ventricular end diastolic diameter(cm) | 6.1±1.1 | 6.1±1.1 | 6.3±1.1 | 0.03 |
| Baseline left ventricular end systolic diameter(cm) | 5.2±1.2 | 5.1±1.2 | 5.3±1.2 | 0.04 |
| Ischemic cardiomyopathy | 225(53%) | 136(49%) | 89(60%) | 0.04 |
| Serum hemoglobin (g/dl) | 12.7±1.9 | 12.6±1.9 | 12.9±1.9 | 0.12 |
| Serum creatinine (mg/dl) | 1.4±0.8 | 1.4±0.8 | 1.4±0.7 | 0.75 |
| QRS duration(ms) | 164.5±25.7 | 167.0±24.4 | 159.8±27.3 | 0.007 |
| Left bundle branch block | 193(46%) | 137(50%) | 56(38%) | 0.02 |
| Paced rhythm | 118(28%) | 88(32%) | 30(20%) | 0.01 |
| History of atrial fibrillation (any type) | 229(54%) | 153(55%) | 76(51%) | 0.47 |
| Chronic obstructive pulmonary disease | 67(16%) | 41(15%) | 26(18%) | 0.49 |
| Hypertension | 261(62%) | 172(62%) | 89(60%) | 0.68 |
| Hyperlipidemia | 247(58%) | 162(59%) | 85(57%) | 0.84 |
| History of malignancy | 63(15%) | 40(15%) | 23(16%) | 0.78 |
| Diabetes mellitus | 158(37%) | 105(38%) | 53(36%) | 0.67 |
| History of stroke or transient ischemic attack | 50(12%) | 26(9%) | 24(16%) | 0.06 |
| Beta-adrenergic blocker | 336(79%) | 220(80%) | 116(78%) | 0.50 |
| Angiotensin converting enzyme inhibitor or angiotensin II receptor blocker | 322(76%) | 215(78%) | 117(79%) | 1.0 |
| Diuretic | 329(78%) | 213(77%) | 116(78%) | 1.0 |
| Anti-arrhythmic medications | 82(19%) | 48(17%) | 34(23%) | 0.20 |
| Nitrates | 120(28%) | 76(28%) | 44(30%) | 0.73 |
| Hydralazine | 51(12%) | 33(12%) | 18(12%) | 1.0 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


