The aim of this study was to evaluate whether a scoring system integrating clinical, electrocardiographic, and echocardiographic measurements can predict left ventricular reverse remodeling after cardiac resynchronization therapy (CRT). The derivation cohort consisted of 162 patients with heart failure implanted with a CRT device. Baseline clinical, electrocardiographic, and echocardiographic characteristics were entered into univariate and multivariate models to predict reverse remodeling as defined by a ≥15% reduction in left ventricular end-systolic volume at 6 months (60%). Combinations of predictors were then tested under different scoring systems. A new 7-point CRT response score termed L 2 ANDS 2 : Left bundle branch block (2 points), Age >70 years, Nonischemic origin, left ventricular end-diastolic Diameter <40 mm/m 2 , and Septal flash (2 points) was calculated for these patients. This score was then validated against a validation cohort of 45 patients from another academic center. A highly significant incremental predictive value was noted when septal flash was added to an initial 4-factor model including left bundle branch block (difference between area under the curve C statistics = 0.125, p <0.001). The predictive accuracy using the L 2 ANDS 2 score was then 0.79 for the C statistic. Application of the new score to the validation cohort (71% of responders) gave a similar C statistic (0.75). A score >5 had a high positive likelihood ratio (+LR = 5.64), whereas a score <2 had a high negative likelihood ratio (−LR = 0.19). In conclusion, this L 2 ANDS 2 score provides an easy-to-use tool for the clinician to assess the pretest probability of a patient being a CRT responder.
The aim of the present study was to evaluate whether a scoring system integrating clinical, electrocardiographic, and echocardiographic characteristics could help the clinician in assessing the probability of left ventricular (LV) reverse remodeling after cardiac resynchronization therapy (CRT). Observational studies suggested that the assessment of mechanical dyssynchrony might be used to help identify CRT responders. New echocardiographic parameters such as septal flash (SF) have been proposed to predict response to CRT with good sensitivity and specificity. This abnormal interventricular septal motion, demonstrated in earlier animal experiments, is seen in some patients with left bundle branch block (LBBB). It can be assessed visually during the isovolumetric contraction, and the clinical value of this motion has been demonstrated recently. The present study evaluates the incremental advantage of a dyssynchrony parameter over clinical and electrocardiographic characteristics in a scoring system.
Methods
This study was conducted in 2 phases. The first one was the score determination. The derivation cohort constituted 162 patients referred to Rennes University Hospital (Rennes, France) from 2010 to 2012 for implantation of a CRT device. To be included in the study, first, the quality of the ultrasound windows was checked. If these windows were judged inadequate, then the patient was not included in the study. Twenty-eight patients were thus excluded. The etiology of dilated cardiomyopathy was ischemic in 35% and nonischemic in 65% of them. The etiology was classified as ischemic if patients either had a history of myocardial infarction or revascularization or showed angiographic evidence of multiple- or single-vessel disease along with left main or proximal left anterior damage. The New York Heart Association (NYHA) class considered was the highest functional class reached. At the time of inclusion, all patients received optimized medical therapy.
The second phase was the score validation. The validation cohort included 45 patients with heart failure referred to Saint Philibert Catholic University Hospital (Lille, France) for implantation of a CRT device in 2011. Five patients were excluded before inclusion because of a poor acoustic window.
At 6-month follow-up, all the patients were reassessed by echocardiography to determine LV reverse remodeling. Responders were defined as those with ≥15% decrease in LV end-systolic volume (LVESV) compared with baseline. The choice of this remodeling end point is the one used in REVERSE (REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction), and its clinical value has been underlined recently.
This study was performed in accordance with the principles outlined in the Declaration of Helsinki on research in human subjects and with the procedures of the local medical ethics committee.
The 12-lead surface electrocardiograms (ECG) were recorded at 25 and 50 mm/s during spontaneous rhythm before implantation of the CRT device and analyzed by Rennes University’s Core Center. The method used for QRS duration analysis has been already reported. Morphology was classified as either LBBB or non-LBBB (including right bundle branch block and nonspecific intraventricular conduction delay). LBBB was defined as a QRS duration of ≥120 ms; QS or rS in lead V 1 ; broad R waves in leads I, aVL, V 5 , or V 6 ; and absent q waves in leads V 5 and V 6 . QRS duration and morphology were measured and assessed by 2 clinical experts.
Baseline echocardiography was performed for each patient before CRT implantation (Vingmed System 7 or e9; GE, Horten, Norway). Digital, routine, gray-scale, 2-dimensional, Doppler, and tissue Doppler imaging cineloops from 3 consecutive cardiac cycles were obtained from the apical views (gray-scale frame rate ≥50 Hz; color frame rate >100 Hz; and 2-, 3-, and 4-chamber apical views). Off-line analyses were performed blindly on digitally stored images (BT12-EchoPAC PC; GE Healthcare). The echocardiographic examination was conducted according to the American Society of Echocardiography guidelines. LV volumes and ejection fraction were calculated using the biplane modified Simpson method. All measurements were averaged for 3 cardiac cycles. LV preejection interval and aortic valve closure were determined using aortic Doppler profiles. The interventricular mechanical delay was calculated as the time difference between the LV and the right ventricle preejection periods. Atrioventricular dyssynchrony was characterized by the LV filling time divided by the RR interval.
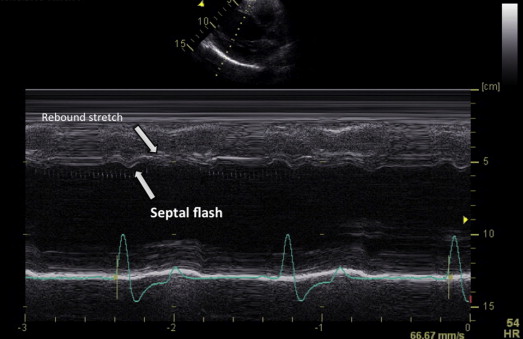
The abnormal interventricular septal motion is seen echocardiographically in some patients with LBBB. They demonstrate leftward displacement of the interventricular septum during preejection, followed by rightward (paradoxical) motion. It has been referred to as an SF. In our study, it was defined as an early septal thickening/thinning within the isovolumetric contraction period as detected both visually from the gray-scale short axis view (SAX) and 4 chamber view (4CH) views and from the parasternal long axis view (PSLAX), SAX, and 4CH views obtained by M-mode ( Figure 1 ). Basal, mid, and apical septal segments were checked. Two clinical experts visually assessed the presence of SF, which was validated if both were in agreement. Patients were categorized according to the presence or absence of SF. All the echocardiographic measurements were done at the echographic core laboratory (CIC-IT 804, CHU Rennes). The analysis of the echorecording was blinded from the clinical status and was performed according to a random way. That means that the 6 months could have been analyzed before the baseline and absolutely not consecutively. Most of the readings were done by 2 cardiologists level 3 in echocardiography.
Indications for CRT device implantation were based on the 2010 Focused Update of European Society of Cardiology guidelines for device use in heart failure therapy (patients in NYHA functional class III or ambulatory class IV with LV ejection fraction ≤35% and QRS duration ≥120 ms or alternatively, patients in NYHA class II with LV ejection fraction ≤35% and QRS duration ≥150 ms). In all patients, the implantation procedure was performed during the month following the qualifying echocardiography. When required, patients received an implantable cardiac defibrillator. Devices were implanted by a single left pectoral incision with transvenous LV lead insertion into a coronary sinus vein, with LV and right ventricular lead placement left to the discretion of the physician.
Patients’ characteristics are given as percentages and means ± SD. Statistical analysis was carried out using the software Statview 5.0 (Abacus Concepts, Berkeley, California) and Medcalc, version 9.3.0 (MedCalc Software, Mariakerke, Belgium). Chi-square tests were used to compare categorical variables between the 2 groups, and the 2 groups’ continuous variables were compared, when appropriate, using the Student t test or the nonparametric Kruskal-Wallis test. A p value <0.05 was considered statistically significant. All potential factors of positive response to CRT identified from the univariate analyses of the derivation cohort with a p value <0.1 were used in the multivariate logistic regression analyses. Age >70 years was a priori imposed in the multivariate model as that age factor is a well-known contributor to higher morbidity and mortality rates in patients with heart failure. Variables with p <0.1 in the final model were considered to be possible contributors. For each of the variables in the final model, the regression coefficient, net odds ratio with its 95% confidence interval, and p values were reported. Different scoring systems were then proposed including SF (termed LANDS) or not (termed LAND). LANDS is an acronym for LBBB morphology on ECG, Age ≥70 years, Nonischemic origin of the cardiomyopathy, LV end-diastolic Diameter indexed (LVEDD) <40 mm/m 2 and SF. We thus tested 4 different scoring systems with different calibrations of LBBB and SF according to the multivariate analysis. Model discrimination and calibration were assessed with C statistics and with the Hosmer-Lemeshow test, respectively. We then calculated the C statistic as a measurement of the predictive accuracy of the model incorporating CRT response factors (with regard to LV reverse remodeling) from the derivation cohort. For each scoring system, the C statistics were calculated and then compared with others using the DeLong test. Sensitivity, specificity, and likelihood ratios of the LANDS score were calculated. To validate the predictive accuracy of our new CRT response scoring system (LANDS), we then tested it on the validation cohort from Lille.
Results
Considering the score determination, baseline characteristics of the 162 patients of the derivation cohort are listed in Table 1 . All patients were in sinus rhythm. One hundred twenty-eight patients (78%) were in NYHA functional class III. QRS duration was 162 ± 25 ms, and 68% of the patients had a typical LBBB morphology on ECG. All patients were on stable, maximally tolerated, heart failure medication: 93% with an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker, 92% with a β blocker, and 88% with a diuretic treatment. A follow-up was obtained for all patients at 7.4 ± 2.7 months. Ninety-eight patients (60%) showed an LVESV reduction ≥15% (responders), whereas 64 (39%) showed either increased LVESV or a reduction in LVESV <15% (nonresponders). Mean LVESV reduction in responders was 41 ± 17%. Mean LVESV change in nonresponders was +3 ± 13%. Baseline characteristics in responders and nonresponders in the derivation cohort are listed in Table 2 . Considering SF assessment, the concordance between reviewers was >90%. Of the 154 patients with both information on QRS morphology and the presence or absence of an SF, we observed an SF in 26 patients (25%) who did not have an LBBB morphology, whereas 78 patients (75%) with an SF had an LBBB morphology. Twenty-seven patients (26%) with an LBBB morphology did not present an SF echocardiographically.
| Variable | All Patients ( n = 207) | Derivation Cohort ( n = 162) | Validation Cohort ( n = 45) | p-Value |
|---|---|---|---|---|
| Age (years) | 66 ± 10 | 65 ± 10 | 68 ± 12 | 0.10 |
| Men | 144 (70%) | 120 (74%) | 24 (53%) | 0.008 |
| Maximum New York Heart Association functional class II/III/IV | 53/152/2 | 34/128/0 | 19/24/2 | 0.0008 |
| Non-ischemic cardiomyopathy | 134 (65%) | 105 (65%) | 29 (64%) | 0.96 |
| Heart rate (bpm) | 67 ± 14 | 66 ± 14 | 71 ± 13 | 0.03 |
| QRS duration (ms) | 161 ± 25 | 162 ± 25 | 156 ± 27 | 0.16 |
| Left bundle branch block morphology | 142 (69%) | 110 (68%) | 32 (71%) | 0.68 |
| N-terminal B-type natriuretic peptide (ng/L) | 2551 ± 2624 | 2551 ± 2624 | – | – |
| Left ventricular ejection fraction (%) | 27 ± 6 | 27 ± 6 | 26 ± 4 | 0.43 |
| Left ventricular end-diastolic diameter (mm/m 2 ) | 37 ± 5 | 37 ± 5 | 37 ± 6 | 0.73 |
| Left ventricular end-diastolic volume (mL) | 232 ± 71 | 231 ± 72 | 238 ± 66 | 0.52 |
| Left ventricular end-systolic volume (mL) | 172 ± 59 | 170 ± 61 | 177 ± 51 | 0.49 |
| Left ventricular pre-ejection interval (ms) | 145 ± 39 | 137 ± 37 | 173 ± 33 | <0.0001 |
| Left ventricular filling time/RR (% RR) | 43 ± 10 | 44 ± 10 | 40 ± 11 | 0.02 |
| Inter-ventricular mechanical delay (ms) | 44 ± 27 | 42 ± 26 | 50 ± 27 | 0.11 |
| Septal flash | 132 (67%) | 104 (68%) | 28 (64%) | 0.63 |
| Variable | Derivation Cohort | Validation Cohort | ||||
|---|---|---|---|---|---|---|
| CRT Responders ( n = 98) | CRT Nonresponders ( n = 64) | p-Value | CRT Responders ( n = 32) | CRT Nonresponders ( n = 13) | p-Value | |
| Age (years) | 65 ± 10 | 65 ± 9 | 0.90 | 69 ± 11 | 67 ± 13 | 0.70 |
| Men | 66 (67%) | 54 (84%) | 0.02 | 14 (44%) | 10 (84%) | 0.04 |
| Maximum New York Heart Association functional class II/III/IV | 22/76/0 | 12/52/0 | 0.65 | 13/18/1 | 6/6/1 | 0.76 |
| Non-ischemic cardiomyopathy | 73 (74%) | 32 (50%) | 0.001 | 21 (66%) | 8 (62%) | 0.80 |
| Heart rate (bpm) | 66 ± 14 | 65 ± 13 | 0.65 | 70 ± 13 | 73 ± 11 | 0.53 |
| QRS duration (ms) | 162 ± 21 | 162 ± 30 | 0.97 | 159 ± 24 | 150 ± 33 | 0.30 |
| Left bundle branch block morphology | 77 (78%) | 33 (51%) | 0.0003 | 24 (75%) | 8 (62%) | 0.37 |
| N-terminal B-type natriuretic peptide (ng/L) | 2595 ± 2773 | 2479 ± 2380 | 0.81 | – | – | – |
| Left ventricular ejection fraction (%) | 27 ± 6 | 27 ± 7 | 0.67 | 27 ± 3 | 24 ± 3 | 0.003 |
| Left ventricular end-diastolic diameter (mm/m 2 ) | 36 ± 4 | 37 ± 6 | 0.33 | 36 ± 6 | 38 ± 6 | 0.42 |
| Left ventricular end-diastolic volume (mL) | 228 ± 68 | 236 ± 77 | 0.51 | 232 ± 66 | 255 ± 67 | 0.31 |
| Left ventricular end-systolic volume (mL) | 168 ± 58 | 172 ± 66 | 0.70 | 169 ± 48 | 195 ± 56 | 0.12 |
| Left ventricular pre-ejection interval (ms) | 144 ± 33 | 125 ± 39 | 0.001 | 176 ± 33 | 165 ± 33 | 0.31 |
| Left ventricular filling time/RR (% RR) | 41 ± 9 | 48 ± 9 | <0.0001 | 39 ± 9 | 41 ± 16 | 0.66 |
| Inter-ventricular mechanical delay (ms) | 46 ± 26 | 37 ± 26 | 0.03 | 56 ± 24 | 35 ± 30 | 0.02 |
| Septal flash | 76 (78%) | 28 (44%) | <0.0001 | 24 (77%) | 4 (31%) | 0.003 |
Univariate regression analysis identified 4 variables with p <0.1 after evaluation of all the clinical, electrocardiographic, and echocardiographic variables. Table 3 lists the findings of univariate and the multivariate regression analyses, which identified 5 variables as potential contributors: age >70 years, nonischemic origin, LBBB, LVEDD <40 mm/m 2 , and SF. Four different scoring systems were then generated from these 5 variables: LAND (LBBB morphology on ECG, Age ≥70 years, Nonischemic origin of the cardiomyopathy, and LVEDD <40 mm/m 2 ); L 2 AND (2 points awarded for LBBB morphology on ECG); LANDS (LBBB morphology on ECG, Age ≥70 years, Nonischemic origin of the cardiomyopathy, LVEDD <40 mm/m 2 , and SF), and L 2 ANDS 2 (2 points awarded for LBBB morphology on ECG and for SF). Comparisons of the predictive accuracy (C statistics) in the derivation cohort for each scoring system are displayed in Table 4 . The predictive accuracy improved significantly when adding SF to the scoring points awarded to LBBB and SF. Figure 2 presents analysis by receiver operating characteristic curves of the different scoring systems in the derivation cohort.
| Risk Factor | Univariable OR (95% CI) | p-Value | Multivariable OR (95% CI) | p-Value |
|---|---|---|---|---|
| Age >70 | 1.52 (0.78–2.95) | 0.220 | 2.22 (0.96–5.12) | 0.062 |
| Female | 2.62 (1.18–5.81) | 0.018 | 1.41 (0.54–3.66) | 0.483 |
| Non-ischemic etiology | 2.92 (1.50–5.68) | 0.002 | 2.30 (1.04–5.10) | 0.040 |
| Left bundle branch block morphology | 3.44 (1.73–6.85) | 0.0004 | 3.18 (1.37–7.37) | 0.007 |
| Left ventricular end-diastolic diameter | 0.97 (0.91–1.03) | 0.326 | 0.92 (0.86–1.00) | 0.045 |
| Septal flash | 5.27 (2.54–10.91) | <0.0001 | 5.16 (2.24–11.86) | 0.0001 |
| Clinical, ECG, and echocardiographic characteristics composing the L 2 ANDS 2 score | ||||
|---|---|---|---|---|
| Letter | Characteristic ∗ | Points Awarded | ||
| L 2 | Left bundle branch block | 2 | ||
| A | Age >70 years old | 1 | ||
| N | Non-ischemic etiology | 1 | ||
| D | Left ventricular end-diastolic diameter <40 mm/m 2 | 1 | ||
| S 2 | Septal flash | 2 | ||
∗ See Methods for definitions of the clinical, electrocardiographic, and echocardiographic characteristics.
| Scoring System | C Statistic ∗ | 95% Confidence Interval |
|---|---|---|
| LAND | 0.648 | 0.567–0.723 |
| L 2 AND | 0.713 | 0.635–0.783 |
| LANDS | 0.773 | 0.699–0.837 |
| L 2 ANDS 2 | 0.790 | 0.717–0.851 |
| Scoring System | Difference Between Areas | 95% Confidence Interval | Significance Level |
|---|---|---|---|
| LAND vs L 2 AND | 0.065 | 0.017–0.114 | p = 0.009 |
| LAND vs LANDS | 0.125 | 0.066–0.183 | p <0.001 |
| L 2 AND vs L 2 ANDS 2 | 0.077 | 0.022–0.132 | p = 0.014 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


