Chapter 8 Coarctation of the Aorta and Interrupted Aortic Arch
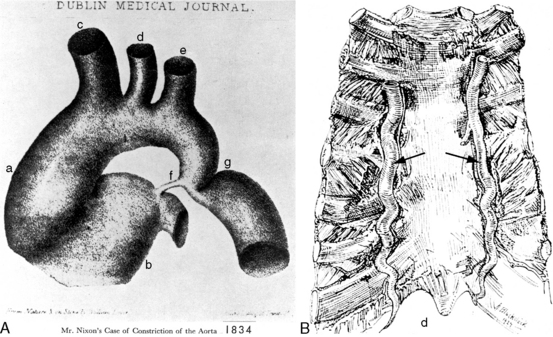
In 1760, the Prussian anatomist, Johann Friedreich Meckel, characterized coarctation of the aorta as an “extraordinary dilatation of the heart which came from the fact that the aortic conduit was too narrow.”1 Saul Jarcho’s1–5 historic papers underscored the accuracy of early accounts of this congenital malformation.
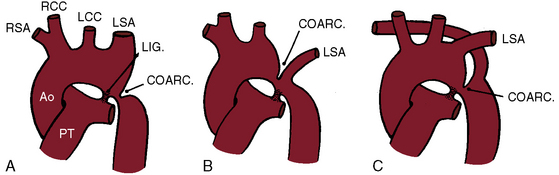
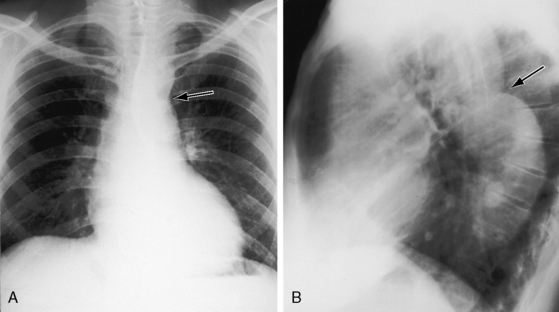
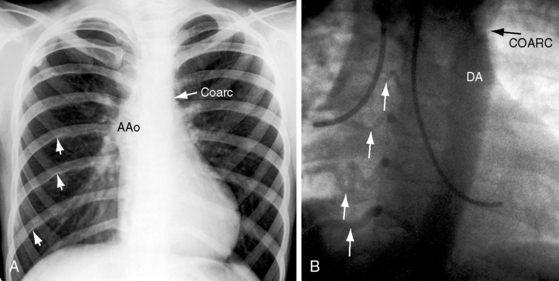
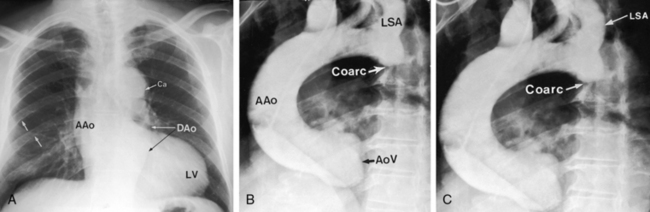
Coarctation of the aorta is typically located near the aortic attachment of the ligamentum arteriosum or patent ductus arteriosus (Figures 8-1A and 8-2). An obtuse indentation in the posterolateral wall of the aorta corresponds to the location of an internal ridge or shelf that eccentrically narrows the aortic lumen, hence the term coarctatus (Latin, contracted or tightened). The ridge that forms the coarctation consists of smooth muscle, fibrous tissue, and elastic tissue similar in composition to a muscular arterial ductus (see Chapter 20).6 Intimal proliferation distal to the ridge narrows the lumen.6 The junction between the ductus and the elastic aorta is clearly defined, with extension of ductal tissue into the aortic wall not exceeding 30% of the aortic circumference. In preductal coarctation, however, ductal tissue forms a circumferential sling that extends around the aorta.7 In juxtaductal coarctation, ductal tissue is not a significant part of the aortic wall.7 The coarctation ridge is thought to represent the original wall of the distal left sixth aortic arch. The neural crest is thought to play a role in the pathogenesis of some types of coarctation.7
The common form of aortic coarctation is represented by a localized constriction that contains the ridge or shelf as just described.6 Less commonly, a relatively long segment of constriction extends beyond the left subclavian artery. Rarely, congenital coarctation is in the mid thoracic aorta.8 Tubular hypoplasia refers to uniform narrowing within the aortic arch.6 Localized coarctation and tubular hypoplasia sometimes coexist.
The mechanisms that account for the typical location of coarctation take into account a number of variables: (1) the quantitative morphology and growth of the aortic arch in the normal fetus9–11; (2) the site of the aortic orifice of the ductus arteriosus; (3) the presence of ductal tissue in the coarctation6; (4) constriction of ductal tissue immediately after birth; and (5) coarctation in the presence of a widely patent ductus in the fetus12,13 and after birth.6 Current consensus favors an interplay between aortic growth and blood flow.9–11 High-resolution echocardiographic imaging in the healthy fetus has disclosed progressive tapering of the diameter of the aortic arch at all gestational ages, with the smallest diameter at the isthmus.9 Tapering is thought to reflect the relative proportion of fetal cardiac output traversing each aortic segment. The smallest proportion traverses the isthmus, which maintains its smaller dimension relative to the remainder of the aortic arch well into postnatal life, especially in premature infants.9 Neonatal coarctation is characterized by hypoplasia of the transverse aorta in the presence of a relatively large pulmonary trunk, a combination that is thought to reflect an in utero decrease in aortic arch flow together with an increase in flow through the main pulmonary artery and ductus.11
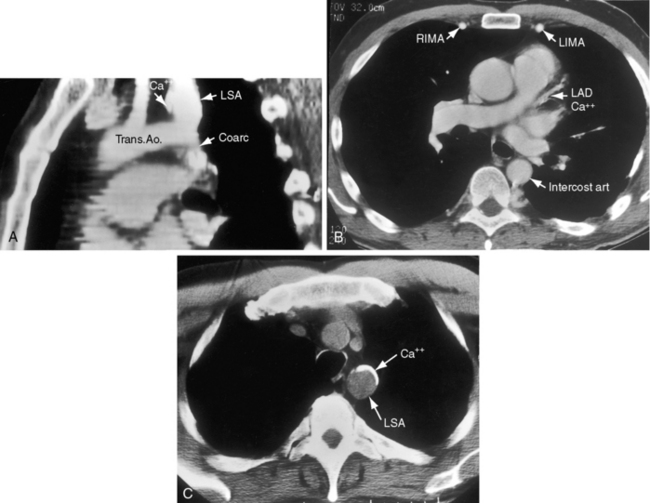
The coarctation ridge is located either immediately proximal to the aortic insertion of the ductus, opposite the aortic insertion (juxtaductal), or immediately distal to the aortic insertion.6,14,15 Infants with a juxtaductal coarctation show no signs of aortic obstruction as long as the ductus is widely patent, assuring unobstructed pulmonary trunk/ductus/descending aortic continuity. When the ductus closes, the coarctation becomes apparent immediately and dramatically and the femoral pulses disappear. The left subclavian artery is dilated because coarctation is usually located immediately distal to its origin (Figure 8-1A, 8-3D, and 8-4A). However, coarctation may lie at or proximal to the left subclavian orifice, compromising its lumen (Figure 8-1B). Exceptionally, the right subclavian artery arises distal to the coarctation (Figure 8-1C) or the coarctation is in a right aortic arch.16
Coarctation of the abdominal aorta can be part of a noncongenital systemic vascular disorder, such as Takayasu’s arteritis or von Recklinghausen’s disease, but can also be congenital and is therefore appropriate for inclusion here.17–20 Abdominal coarctation is rare, accounting for 0.5% to 2% of all varieties of aortic coarctation,18 and can be suprarenal, infrarenal, or interrenal.18 Systemic hypertension is believed to result from involvement of the renal artery.20 A congenital etiology is based on the combination of a localized hourglass deformity with an intraluminal membrane, occurrence at a young age, absence of a systemic vascular disorder, and slow progression with development of extensive collaterals.20
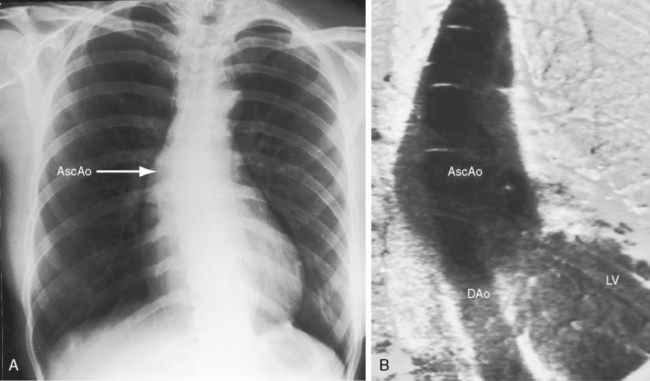
Pseudocoarctation refers to a rare anomaly characterized by buckling or kinking of the aorta in the vicinity of the ligamentum arteriosum that results in elongation, tortuosity, and dilation of the distal aortic arch and the proximal descending aorta.21–25 Occasionally, pseudocoarctation involves the abdominal aorta (Figure 8-5).26 Pseudo signifies absence of a gradient at the site of the localized external deformity, absence of collaterals, and absence of systemic hypertension, although refractory hypertension has been reported.27 Occasionally, transformation from pseudocoarctation to true coarctation occurs.28 The femoral pulses are sometimes reduced because of the damping effect of sharp angulation of the aortic arch (see Figure 8-5).22 Thin-walled saccular aneurysms may form in the distal segment,21 spontaneous rupture and dissection have been reported in the proximal descending thoracic aorta29 and in the ascending aorta,30 and bicuspid aortic valve may coexist.31
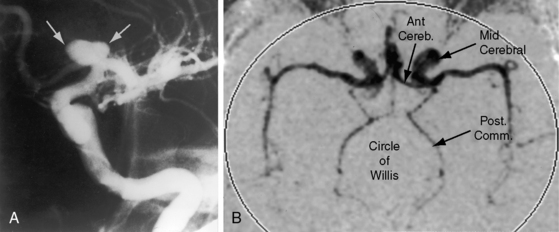
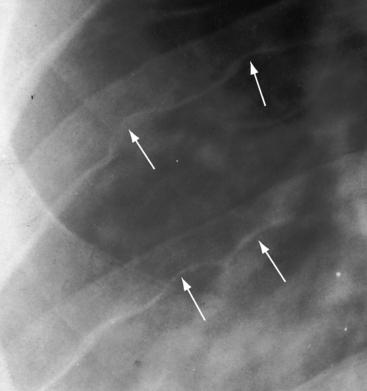
Coarctation of the aorta is usually and simplistically regarded as isolated obstruction of the aortic isthmus32 but in fact is a widespread disorder in which isthmic obstruction is only one of a cluster of abnormalities that include the proximal and distal paracoarctation aorta, the ascending and transverse aorta, the coronary arteries, conduit arteries (radial, brachial, carotid), the retinal vascular bed, dissecting aneurysm, cerebral aneurysms, vascular rings, and systemic hypertension. The strongest association is with a functionally normal, stenotic, or incompetent bicuspid aortic valve (Figure 8-6B; see Chapter 7)33,34 characterized by fusion of the right and left coronary cusps.34,35 A decrease in left ventricular interpapillary muscle distance is common36and culminates in the single papillary muscle of a parachute mitral valve (see Chapter 9).37,38 Coarctation in the fetus tends to be associated with a left superior vena cava.39 Endocardial fibroelastosis is patchy rather than confluent.40 Two shunts accompany coarctation (patent ductus arteriosus41 and ventricular septal defect41–44), which is usually characterized by leftward malalignment that curtails the amount of blood that reaches the aortic isthmus.42–44 Dissecting aneurysm with hemopericardium was reported in 1830.45 Aneurysmal dilation, which can be large, saccular, and calcified, occurs in the low-pressure high-velocity distal paracoarctation segment.46 The substrate for aneurysm formation is an inherent abnormality of medial smooth muscle and extracellular matrix that is a consistent feature of the proximal and distal paracoarctation aorta.47,48 Aneurysms of the circle of Willis set the stage for intracranial hemorrhage (Figure 8-7). Relatively benign aneurysms occasionally develop in intercostal arteries.49 A large aneurysm of the left subclavian artery was acutely dissected.50 The retinal arterioles are characteristically U shaped (see subsequent discussion).51
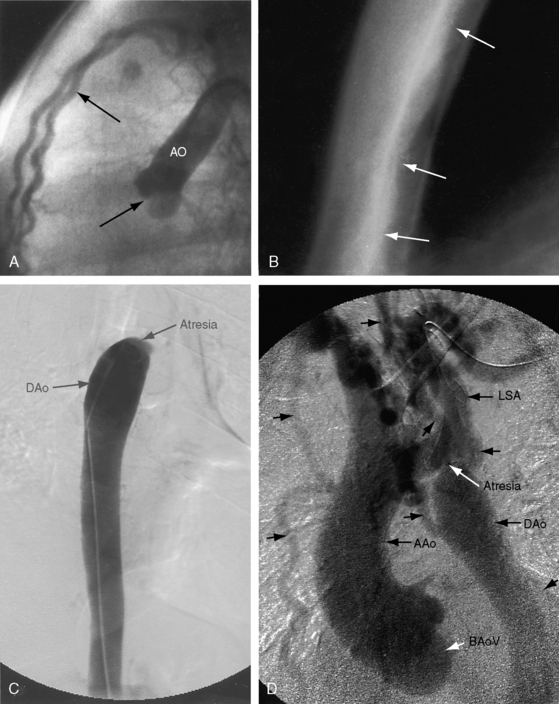
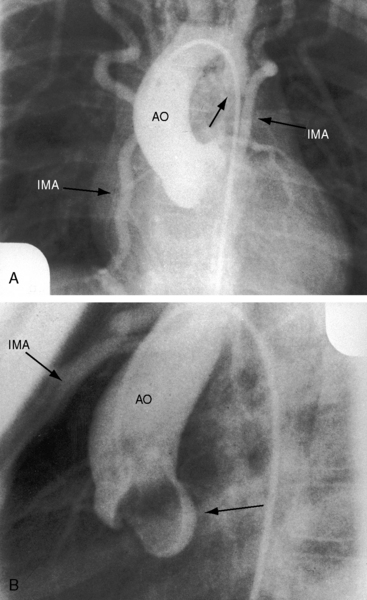
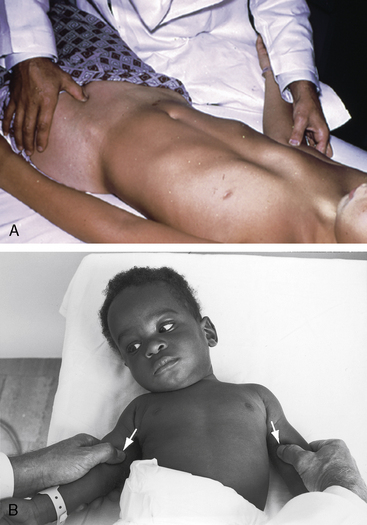
Arterial collaterals are important vascular sequelae of coarctation and depend on subclavian artery patency for their development (see Figures 8-3 and 8-6A). When coarctation obstructs the orifice of the left subclavian artery, ipsilateral collaterals fail to develop (see Figure 8-27). A subclavian steal may result from retrograde flow down the ipsilateral vertebral artery.52 Systemic-to-pulmonary collaterals normally present after birth gradually disappear.53
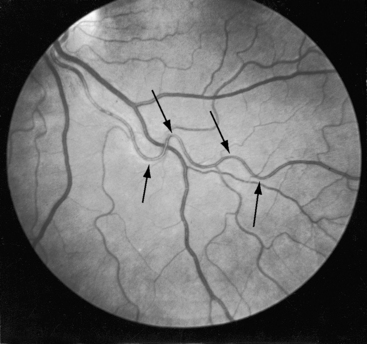
Retinal arterioles are the site of a distinctive vascular pattern.51,54 In 1948, at the annual meeting of the Swedish Ophthalmological Society, Professor K.O. Granstrom55 stated, “As in other cases of hypertension, these patients are sent as a matter of routine for examination to the eye department. As soon as I had seen a few such cases, it became evident that the retinal picture in coarctation of the aorta is often somewhat characteristic, the principal feature being pronounced tortuosity of the arteries.” The U-shaped tortuous retinal arterioles (Figure 8-8) may be accompanied by serpentine pulsations synchronous with the arterial pulse.54 Granstrom55 correctly concluded that the distinctive retinal abnormality in coarctation was benign and that hypertensive retinopathy was conspicuous by its absence.
Coarctation of the aorta can contribute to a vascular ring that consists of a double aortic arch or a right aortic arch, a left ligamentum arteriosum, and an aberrant subclavian artery.56–58 An anomalous retroesophageal right subclavian artery occasionally accompanies coarctation of the aorta (see Figure 8-1C) but does not cause tracheoesophageal compression.
The pathogenesis of systemic hypertension in coarctation of the aorta has been the subject of three theories. The mechanical theory focuses on resistance at the site of obstruction. This theory in itself cannot be sustained but is central to the neural theory that involves the sensitivity of carotid sinus baroreceptors and the distensibility characteristics of the precoarctation aorta.59 The proximal aortic segment of coarctation has an increase in collagen and a decrease in smooth muscle that are responsible for an increase in stiffness and a decrease in distensibility.60 The poorly compliant precoarctation aorta is responsible for the elevation of systolic blood pressure at rest, the disproportionate rise during isotonic exercise,61,62 and the resetting of carotid sinus baroreceptors to operate at higher pressures.59,61 The renal theory of pathogenesis takes into account a unique feature of coarctation hypertension, namely, that the elevated blood pressure is confined to the upper extremities. Accordingly, the renal arteries are exposed to the neurohumeral effects of hypertension but not the hydraulic effects. The coarctation gradient increases during isotonic exercise, which is accompanied by a disproportionate increase in systolic pressure,63 an exaggeration of the inherent disproportionate systolic hypertension related to rigidity of the precoarctation aortic wall. The response of the mean blood pressure to exercise is approximately the same as in control subjects.64 Experimental hypertension has been produced by aortic constriction that assigns the renal arteries to the distal low pressure zone.65 When the constriction is below the renal arteries, blood pressure remains normal.65,66 Toxemia of pregnancy is not a feature of coarctation hypertension. Eye grounds do not exhibit hypertensive retinopathy but instead exhibit distinctive serpentine retinal arterioles (see Figure 8-8).51,54
The increase in left ventricular mass induced by the afterload of coarctation is characterized as myocyte replication rather than myocyte hypertrophy,67 which is thought to account for reduced end-systolic wall stress and enhanced left ventricular ejection performance.68
History
The male:female ratio of coarctation is from 1.4:1 to 3:1.69 Familial recurrence has occurred in siblings, in monozygotic twins, and in parent and child70–75 and as autosomal dominant in four generations.76 True coarctation and pseudocoarctation have been reported in siblings.77 Turner’s syndrome (see section Physical Appearance) cannot be transmitted because of sterility, but seven women in three generations of one family had Turner’s syndrome attributed to loss of the short arm of one X chromosome that permitted transmission from phenotypically normal female carriers with a balanced X-1 translocation.78 A peak seasonal incidence of coarctation is found from September through November and from January through March.79 The reported rarity of coarctation in African Americans is open to question.80,81
Coarctation of the aorta usually produces significant symptoms in early infancy69,82,83 and after age 20 to 30 years.84 Neonates with severe coarctation become acutely symptomatic when the ductus closes. Most who survive the hazards of infancy reach adulthood, although more than a quarter die by age 20 years, half by age 30 years, and more than three quarters by age 50 years.33,84,85 Figures 8-24 and 8-26 are from patients aged 54 years, 62 years, and 70 years. Survival has been reported at age 74 years and 76 years.86 Isolated atresia of the aortic arch was surgically repaired at age 65 years.87 The longest recorded survival was Reynaud’s account (1828) of a 92-year-old man with coarctation.3 The anatomic illustrations in this report are noteworthy. However, examples of exceptional longevity should not obscure the inherent risk of coarctation that results in death at an average age of 33 years.
Mild coarctation is not always benign, and severe coarctation is not always asymptomatic. A case in point, albeit rare, is a 20-year-old woman with severe coarctation, borderline hypertension, and no symptoms.88 Except for symptomatic infants, patients tend to be clinically well when the diagnosis is first made. Initial suspicion requires little more than attention to upper and lower extremity arterial pulses and blood pressure (see section The Arterial Pulse). Nevertheless, delayed recognition is not uncommon,89,90 and diagnoses have been made by chance in the fifth decade.91 Minor symptoms include epistaxis and leg fatigue. When coarctation compromises the orifice of the left subclavian artery in left-handed patients, muscular fatigue involves the left arm. Leg fatigue occurs in about half of patients, but claudication is reserved for abdominal coarctation. Patients are sometimes subjectively aware of amplified arterial pulsations in the neck, especially after effort or excitement. Dysphagia occurs when a retroesophageal right subclavian artery originates distal to the coarctation and passes behind the esophagus (see Figure 8-1C)92 or when coarctation is a component of a vascular ring.
Major symptoms are features of four eventualities: (1) congestive heart failure; (2) rupture or dissection of the aorta; (3) infective endarteritis or endocarditis; and (4) cerebral hemorrhage.83,84,93–95 Hypertension is chiefly responsible for morbidity and mortality with advancing age.83 The incidence rate of cardiac failure is highest in infants69,96 and is high again after the fourth decade.97 In a review of 234 patients with coarctation of the aorta aged 1 day to 72 years, congestive heart failure occurred in two thirds of patients less than 1 year of age and more than age 40 years but in only 4% of patients between 1 year and 40 years of age.83 Many, if not most, neonates and infants with congestive heart failure have a coexisting ventricular septal defect or patent ductus arteriosus.98,99 In brief, more than 90% of infants and children with uncomplicated coarctation experience little or no difficulty.100
When coarctation is juxtaductal or proximal to the neonatal ductus, continuity between the pulmonary trunk and descending aorta is maintained, so the femoral arterial pulses remain palpable.6,96 When the ductus closes, the femoral pulses disappear, pulmonary blood flow is diverted into the lungs, the left ventricle is suddenly volume overloaded, and blood pressure and blood flow proximal to the coarctation suddenly increase. A high left atrial pressure opens the pliant valve of the foramen ovale, initiating a left-to-right shunt that imposes a volume load on the right ventricle that is already pressure overloaded because of pulmonary hypertension.101 Initial closure of the pulmonary arterial end of the ductus can leave the aortic end sufficiently patent to permit the proximal aorta to decompress.15 When the aortic end of the ductus closes, the isthmus is suddenly obstructed. Distal aortic pressure and flow fall, renal perfusion falls, and the renin-angiotensin system is activated.65,102
Rupture or dissection of the aorta is a dramatic complication with peak incidence in the third and fourth decades.83,85,94,97 The rupture originates either in a paracoarctation aneurysm47,48,94 or above a coexisting bicuspid aortic valve because of an inherent medial abnormality of the ascending aorta (see Chapter 7).48,94,97 Rupture of a postcoarctation aneurysm may be accompanied by bleeding into the esophagus that is announced by hematemesis and melena. In XO Turner’s syndrome (see section Physical Appearance), rupture or dissection of an ascending aortic aneurysm occurs because of an inherent medial abnormality,48 whether or not a coexisting coarctation or a bicuspid aortic valve exists (Figure 8-9).103–105 Aneurysms of intercostal arteries are almost always occult and relatively benign.49 Pseudocoarctation has been regarded as a disorder that incurs little or no risk, but the malformation is not necessarily benign and is not necessarily pseudo (see previous discussion). Pseudocoarctation has been reported with Turner’s syndrome.106 Cerebral arterial aneurysms are discussed in the section on cerebrovascular accidents (see subsequent discussion).
Infective endarteritis or endocarditis is a major complication of coarctation of the aorta, although the more susceptible site is a coexisting bicuspid aortic valve (see Chapter 7).83,97 Saccular septic aneurysms are occasional sequelae of infective endarteritis.94
Cerebrovascular accidents are the fourth major eventuality in coarctation of the aorta.83,93,107 Hypertension is not a necessary precondition because cerebral complications can occur with normotensive conditions long after successful repair. An aneurysm of the circle of Willis, first described in 1927,108 is the chief offender (see Figure 8-7) and sets the stage for rupture and cerebral hemorrhage.109 Less common, but not less important, are aneurysms in other cerebral arteries.93 Infective endocarditis on a bicuspid aortic valve can give rise to septic cerebral aneurysms that rupture. Rarely and oddly, an unruptured intracranial aneurysm triggers musical hallucinations.110
Coarctation hypertension is a risk factor for premature atherosclerotic coronary artery disease (see Figure 8-4B; see previous discussion).83,111,112 Evidence also exists of structural abnormalities of terminal intramural coronary arteries.111 Gonadal dysgenesis of Turner’s syndrome reportedly increases atherosclerotic cardiovascular risk.113
Physical Appearance
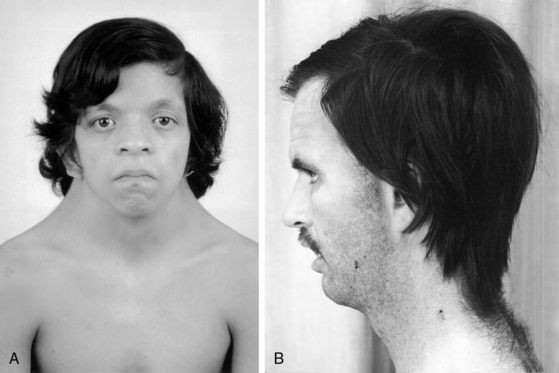
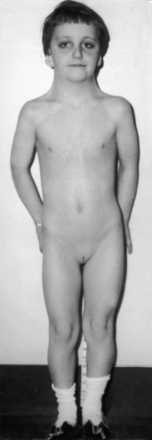
XO Turner’s syndrome with its distinctive physical appearance (Figure 8-10) arouses suspicion of coarctation of the aorta.114,115 Chromosomal patterns 45 XO and X-mosaicism are coupled with additional cardiac expressions.116–119 Aortic rupture or dissection in Turner’s syndrome was mentioned previously (see Figure 8-9).103,104,120 The typical XO Turner’s phenotypic female (Figure 8-11) is of short stature with webbing of the neck, absent or scanty auxiliary and pubic hair (ovarian dysgenesis), broad chest with widely spaced hypoplastic or inverted nipples, low anterior and posterior hairlines, small chin, prominent ears because of large auricles, cubitus valgus, short fourth metacarpals and metatarsals, distal palmar triaxial radii, narrow hyperconvex nails, and pigmented cutaneous nevi.115 Infants with Turner’s syndrome exhibit lymphedema of the neck with loose skin, puffiness of the dorsum of the hands and feet, and low hairlines.115,121 Congenital heart disease is much more frequent in Turner’s syndrome with webbing of the neck than in Turner’s syndrome without webbing,116,121 and coarctation of the aorta is eight times as frequent when Turner’s syndrome is accompanied by webbing of the neck.121 Noonan’s syndrome (Turner’s phenotype with normal genotype; see Figure 8-10) is only occasionally accompanied by coarctation of the aorta122 and rarely by ascending aortic aneurysm.95,115,123 Systemic hypertension in Turner’s syndrome occurs without coarctation. Another distinctive physical appearance associated with coarctation of the aorta is the PHACE syndrome124: P, posterior fossa brain malformations; H, hemangioma of the head or neck; A, arterial abnormalities of the head or neck; C, cardiac abnormalities, including coarctation of the aorta; E, eye abnormalities.
Arterial Pulse
Abnormal differences in upper and lower extremity arterial pulses and blood pressure are hallmarks of coarctation of the aorta. If proper attention were paid to these pulses, few or no diagnoses would be missed.90,125 Two methods have been advocated for comparison of upper and lower extremity arterial pulsations. One method compares femoral and radial pulses; the other method compares femoral and brachial pulses. Normally, radial and femoral pulses are sensed as synchronous, so that any femoral delay is considered abnormal. Positioning the patient’s wrist next to the groin is believed to facilitate comparison. In infants and newborns, the brachial artery is more readily palpated than the tiny radial artery, which is surrounded by subcutaneous fat pads (Figure 8-12B). The author’s preference is comparative palpation of brachial and femoral pulses, which is accomplished with placing a thumb on each (Figure 8-12A).125 The slight delay in perceived arrival time of the normal femoral pulse (15 msec in adults, less in infants and children) is easily sensed as a norm against which even slight deviations can be judged. With palpation of the femoral pulse in an infant, the patient must be allowed to relax the legs voluntarily because forceful restraint can decrease or abolish a normal femoral pulse.
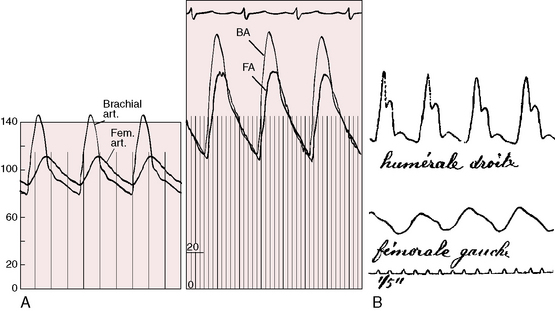
What is perceived as an abnormal delay of the femoral pulse in coarctation is not a delay in arrival time but instead a slow rate of rise to a delayed peak (Figure 8-13). The presence of a normal femoral pulse effectively eliminates all but mild coarctation, provided the aortic valve is functioning normally (see subsequent discussion). Palpation begins with applying to the brachial and femoral arterial pulses the amount of digital pressure necessary to elicit the maximal systolic impact.125 Stiffness of the precoarctation aortic wall results in disproportionate systolic hypertension and forceful carotid and suprasternal notch pulsations, which are sensed by the patient and become increasingly apparent with age and exercise (see previous discussion).59,61,62
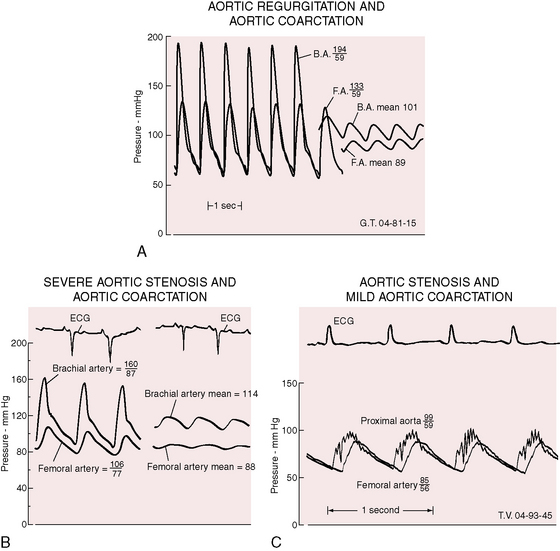
Bicuspid aortic regurgitation reinforces the femoral pulse, which can be misjudged unless properly compared with the brachial pulse (Figure 8-14A).126 Bicuspid aortic stenosis may not dampen the brachial arterial pulse because coarctation amplifies the ascending aortic and brachial arterial systolic pressures (Figure 8-14B).126 Conversely, if aortic stenosis dampens the ascending aortic systolic pressure, evidence of coarctation is obscured (Figure 8-14C).126
The right and left brachial arteries should be compared with palpation and with cuff blood pressure (see Figure 8-12B). A decreased or absent left brachial arterial pulse indicates that the coarctation has compromised the lumen of the left subclavian artery (see Figure 8-1B) or is proximal to its origin (see previous discussion). In comparison of the two brachial pulses, account must be taken of normal systolic pressure differences of 10 to 15 mm Hg lower in the left arm.125 Diminution or absence of the right brachial pulse implies that the right subclavian artery originates distal to the coarctation (see Figure 8-1C). Absence of both brachial arterial pulses signifies that coarctation is compromising the lumen of the left subclavian artery and that the right subclavian artery arises distal to the coarctation.127 At birth, femoral arterial pulses are temporarily palpable if the coarctation is juxtaductal, if the ductus is distal to the coarctation, or if the aortic end of the ductus is patent.6 The abdominal aorta is readily palpable proximal to abdominal coarctation but is not palpable when coarctation is in the aortic isthmus. Pseudocoarctation leaves the arterial pulses normal because there is no aortic obstruction, although angulation at the level of the ligamentum arteriosum occasionally dampens the femoral pulses (see Figure 8-5).22
Collateral arterial pulsations are specifically sought with patients who are old enough standing and bending forward with arms hanging at the sides while the examiner scrutinizes the patient’s back, especially around and between the scapulae.128 A tangential light in a darkened room highlights subcutaneous collaterals in shadowed relief. Collateral arteries occasionally appear around the shoulders, along the right and left sternal borders, and rarely over the upper abdominal wall.
Cuff blood pressure is an important supplement to palpation of the arterial pulses. Blood pressure should be taken in the right arm, left arm, and leg. Correct cuff size is essential because improper size results in inaccurate readings.125,129–133 An undersized cuff overestimates and an oversized cuff underestimates intraarterial blood pressure by as much as 10 to 30 mm Hg.133 The American Heart Association recommendations for cuff size130 are shown in Table 8-1. References to cuff size apply to the width and length of the inner inflatable bladder, not to the cloth covering. Cuff size is determined by limb circumference, not by patient age. The width of the inflatable bladder within the cuff should be 40% of the circumference of the midpoint of the limb. The length of the inflatable bladder must be sufficient to encircle the limb without overlapping.129,130
Table 8-1 Recommended Bladder Dimensions for Blood Pressure Cuff
| Arm Circumference at Midpoint* (cm) | Bladder Width (cm) | Bladder Length (cm) |
|---|---|---|
| 5-7.5 Newborn | 3 | 5 |
| 7.5-13 Infant | 5 | 8 |
| 13-20 Child | 8 | 13 |
| 17-26 Small adult | 11 | 17 |
| 24-32 Average adult | 13 | 24 |
| 32-42 Large adult | 17 | 32 |
| 42-50 Thigh | 20 | 42 |
* Midpoint is defined as half the distance from the acromion to the olecranon.
From Kirkendall WM, Feinleib M, Freis ED, Mark AL. AHA committee report: recommendations for human blood pressure determinations by sphygmomanometers. Circulation 1980;62:1146A. Reprinted with permission of the American Heart Association.
The examining room should be quiet, and the patient must be comfortable and reassured. Sufficient time should elapse for recovery from recent activity and tension. When an infant is quieted with a bottle or a pacifier, blood pressure can be obtained with the usual auscultatory method. An alternative is the flush technique, which requires two examiners.129 An uninflated cuff is applied to the infant’s forearm, which is elevated while the limb distal to the cuff is massaged to induce blanching. The cuff is inflated above anticipated systolic pressure while the arm remains elevated and blanched. The arm is then slowly lowered into a horizontal position while the cuff is slowly deflated. The point at which the blanched hand becomes flushed is an estimate of the mean arterial pressure. Doppler ultrasound or oscillometric techniques can be used by a single examiner and accurately determine systolic and diastolic blood pressure.90,129
Upper extremity blood pressure in infants and adults is usually recorded in the supine position, but a comfortable sitting position in a parent’s lap is acceptable for relaxation of infants and young children. Lower extremity blood pressure is best determined from the popliteal artery with the patient prone.125 While the popliteal artery is palpated, a thigh cuff is applied and must be inflated slowly to avoid discomfort. The cuff is inflated to a level just above the brachial arterial systolic pressure until the popliteal pulse vanishes. Systolic and diastolic pressures are then estimated with auscultation of popliteal Korotkoff’s sounds. Diagnostic differences in arm and leg blood pressures are based on systolic levels when only systolic Korotkoff’s sounds are heard. However, the diastolic pressures are important in confirmation of accuracy because they are not significantly different in the upper and lower extremities (see Figure 8-13). When intraarterial systolic, diastolic, and mean brachial and femoral pressures are compared in healthy persons, no significant difference is found, although the femoral auscultatory systolic pressure is about 10 mm Hg higher than the direct femoral arterial pressure.133 Differences between arm and leg systolic pressures in coarctation are exaggerated by exercise, which causes an increase in brachial arterial pressure, with no change or a reduction in femoral arterial pressure.134
Measurements of blood pressure should be a routine part of the physical examination after infancy and are desirable in infants at least once.90,135 Normal arterial pressure is based on age and gender.129,136,137 The average systolic blood pressure in females rises to a plateau at around age 14 years and remains almost constant during the early reproductive years.136 The average systolic blood pressure in males may not plateau until around age 20 years.129,136,137 Diastolic pressure differences are in the same direction but smaller in degree.
From birth to 6 months, normal upper extremity blood pressure averages 80/45 mm Hg. From 2 years to puberty, the average upper extremity blood pressure is 90/60 to 100/60 mm Hg,129,136 with the diastolic pressure more consistent than the systolic pressure. Coarctation of the aorta is accompanied by a progressive age-related increase in blood pressure, especially systolic, so the pulse pressure widens.61,73
In pregnant females with coarctation, blood pressure fluctuations are similar in direction to fluctuations in uncomplicated pregnancy but occur from a higher baseline.138 Coarctation is not a resistance vessel disease, so the incidence rate of toxemia is lower than in pregnant women with other forms of hypertension.138,139 Gestational hypertension is related to a significant coarctation gradient.140 Pregnancy increases the risk of aortic rupture and intracranial hemorrhage because gestational changes in connective tissue reinforce the medial abnormalities inherent in the arterial walls of coarctation.48,138 In abdominal coarctation, hypertension is likely to be caused by renal artery stenosis rather than by the coarctation.20
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree