COARCTATION OF THE AORTA
Introduction
Coarctation of the aorta (CoA), apart from being the most important treatable cause of secondary hypertension later in life, can present with severe symptoms in the neonatal period. The prevalence of CoA is between 5% and 8% of all congenital heart defects (CHDs),1,2 but it is more frequently seen in symptomatic neonates.3 CoA is defined as a CHD consisting of a narrowed aortic segment comprising of localized medial thickening with infolding of the media and superimposed neointimal tissue.4 This localized constriction may form a shelf-like structure with an eccentric opening or a membranous curtain-like structure with a central or eccentric opening. The CoA may be a discrete narrowing, or a long segment of the aorta may be involved; the discrete form is more common. The classic CoA is located in the thoracic aorta distal to the origin of the left subclavian artery (LSA) and is juxtaductal. Varying degrees of hypoplasia of the transverse aortic arch (TAA) (between the origin of the right innominate [RInn] artery and LSA) and of the isthmus of the aorta (aorta between the origin of the LSA and ductus arteriosus) are present, particularly in the symptomatic neonate and infant. Dilatation of the descending aorta (DAo) immediately distal to the coarctation segment, poststenotic dilatation, is usually seen. Collateral vessels that connect arteries from the upper part of the body to the vessels below the level of coarctation are seen in children and are not usually seen in the neonate.
CoA is associated with clinically significant CHD particularly in the neonate and these include patent ductus arteriosus (PDA), ventricular septal defect (VSD) and aortic stenosis. Bicuspid aortic valve may be seen in two-third of infants with CoA. Mitral valve anomalies are less frequent than those of the aortic valve, but are also seen. At times, CoA is a complicating feature of a more complex, cyanotic CHD, such as double-inlet left ventricle, Taussig–Bing anomaly, tricuspid atresia with transposition of the great arteries (TGA) and hypoplastic left heart syndrome (HLHS). Aortic coarctation is rare to be seen in patients with severe right ventricular outflow tract (RVOT) obstructions such as tetralogy of Fallot (TOF) and pulmonary atresia with intact ventricular septum (PA-IVS). The CoA is the most common cardiac defect seen in babies with Turner’s syndrome.
The mechanism by which aortic coarctation is formed is not known. The most commonly proposed hypotheses are hemodynamic and ectopic ductal tissue theories. In the hemodynamic hypothesis, an abnormal preductal flow is deemed to cause coarctation. Spontaneous closure of the ductus arteriosus after birth completes the development of aortic narrowing.4–6 An increased prevalence of CoA in CHD babies with decreased antegrade aortic flow in utero and virtual absence of CoA in patients with right heart obstructive lesions do support the hemodynamic theory. Anomalous extension of ductal tissue onto the aortic wall (ectopic ductal tissue)6–8 has been put forward to explain creation of coarctation when the ductal tissue constricts. This explanation, however, does not account for variable degrees of isthmic arch and aortic arch hypoplasia seen in many babies with CoA.
Clinical Features
By and large the extent of patency of the ductus arteriosus and the rapidity of ductal closure determine the timing of clinical presentation and severity of symptoms. In addition, associated heart defects and aortic arch anomalies as well as the level of pulmonary vascular resistance influence the clinical features.
Neonates and young infants typically present with signs of heart failure such as tachypnea, dyspnea, tachycardia, hepatomegaly, and rales in the chest. When the baby is in heart failure, all pulses are decreased. Following treatment of heart failure, prominent brachial pulses with weak or nonpalpable femoral arterial pulses may be seen. Precordial examination reveals increased left and right ventricular impulses and an ejection systolic murmur in patients with isolated coarctation. At times, there may be no murmur heard. In the presence of a VSD a holosystolic murmur at the left lower sternal border is auscultated. If aortic stenosis is present, an ejection systolic murmur at the right upper sternal border, radiating to the carotid arteries, may be heard. A continuous murmur of PDA is rarely, if ever heard. It is highly important to carefully measure blood pressure in all 4 extremities. A higher arm pressure than leg pressure (greater than 20 mmHg) is usually seen. Sometimes blood pressure difference may not be discerned either because of ductal right-to-left shunt or because of decreased cardiac output. Failure to thrive may be present in older babies.
The babies that present with symptoms later in the neonatal period have been postulated to develop aortic obstruction more gradually than in neonates.5
Noninvasive Evaluation
Chest X-Ray
Chest roentgenogram may reveal cardiomegaly and increased pulmonary vascular markings. Pulmonary venous congestion may also be seen, especially in babies with cardiac failure. Rib-notching secondary to collateral vessels seen in older children and adults is not seen in the neonate.
ECG
In the neonate, the ECG frequently shows right ventricular hypertrophy. The reasons for right ventricular preponderance in the face of left ventricular outflow obstruction is not clearly understood. Rapid constriction of the ductus arteriosus producing sudden, severe aortic obstruction may be the most likely explanation. As the aortic end of the ductus constricts, the left ventricular afterload rapidly increases, which results in increase of left ventricular systolic and diastolic pressures. This in turn results in elevation of the left atrial pressure, which may open the foramen ovale (FO), causing left-to-right shunt, and dilatation of right atrium and right ventricle. Or, if the FO did not open, elevation of pulmonary venous pressures with consequent increase in pulmonary artery (PA) and right ventricular pressure will successively ensue, explaining the right ventricular hypertrophy. Thus, the cardiomegaly on a chest x-ray and right ventricular hypertrophy seen on ECG and echocardiogram appear to be related to the indirect effects of rapid development of severe aortic obstruction.
Echocardiogram
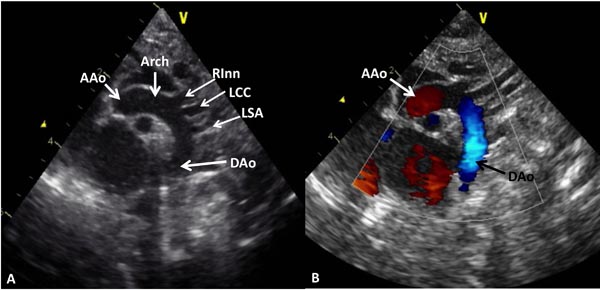
Suprasternal notch, 2-dimensional (2D) echocardiographic views along with color flow mapping are helpful in demonstrating normal aortic arch (Figure 38.1). Such imaging is also very useful in demonstrating CoA by 2D imaging (Figures 38.2A and 38.3A), color Doppler flow acceleration (Figures 38.2B and 38.3B) and demonstrating increase in Doppler flow velocity from the isthmus of the aortic arch to the DAo (Figure 38.4). When descending aortic flow velocity at the level of diaphragm is recorded, the flow is damped (Figure 38.5).
Figure 38.1. A: Suprasternal notch view of normal aortic arch (Arch) demonstrating the AAo, aortic arch and DAo. Note the origin of the RInn, left common carotid (LCC) artery and LSA arising from the aortic arch. B: The same view with color Doppler imaging showing red flow in the AAo and blue flow in the DAo.
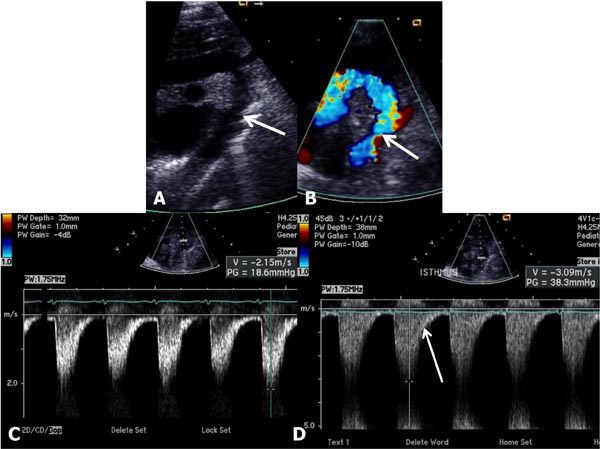
Figure 38.2. Selected video frames from suprasternal notch views in a neonate with aortic coarctation; 2D (A) and color flow (B) images are shown. The arrows point to the site of coarctation. Doppler flow velocity increased from the aortic isthmus (C) to the DAo (D) along with diastolic extension (arrow in D).
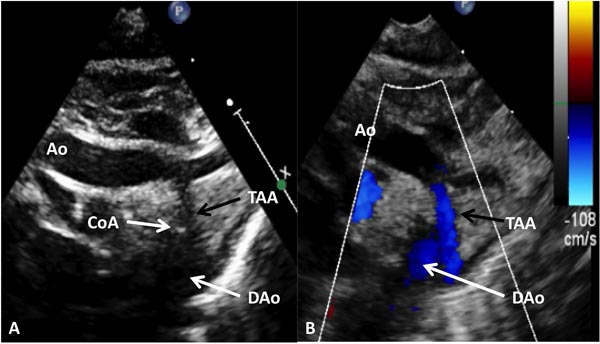
Figure 38.3. Suprasternal notch view of aortic arch (Arch) in a neonate with aortic coarctation (arrow) in 2D (A) and color flow mapping (B) demonstrating turbulence at the site of coarctation (arrow). BCV, brachiocephalic vessels.
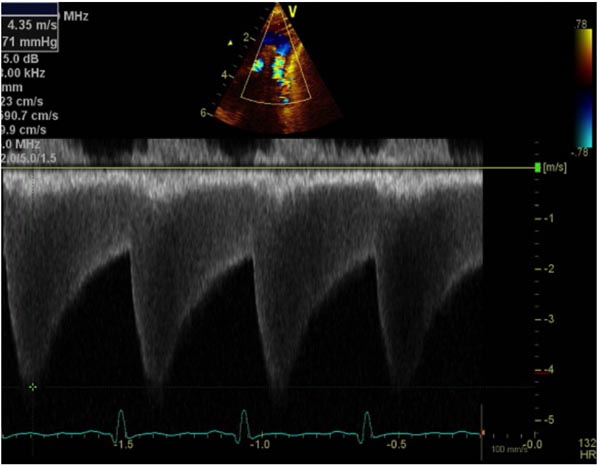
Figure 38.4. Doppler interrogation of the Arch reveals a jump in the peak flow velocity from the proximal segment (A) to the segment distal to coarctation (B). Continuous-wave Doppler interrogation across the stenotic segment (B) shows diastolic extension of the Doppler flow, suggesting that the coarctation is severe.
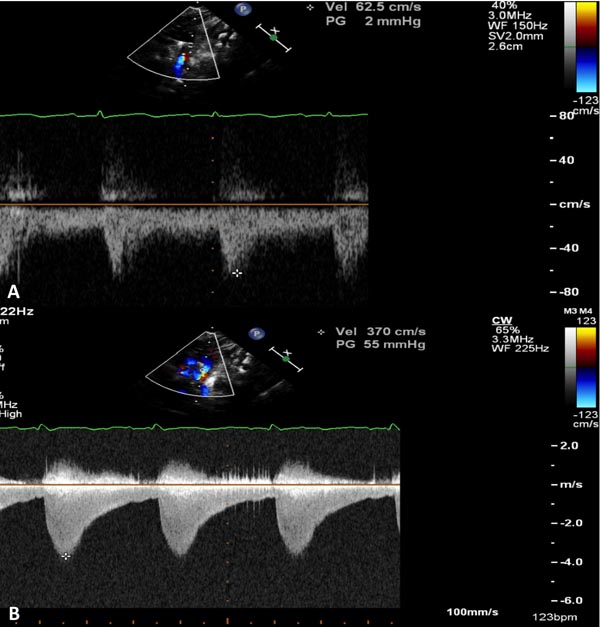
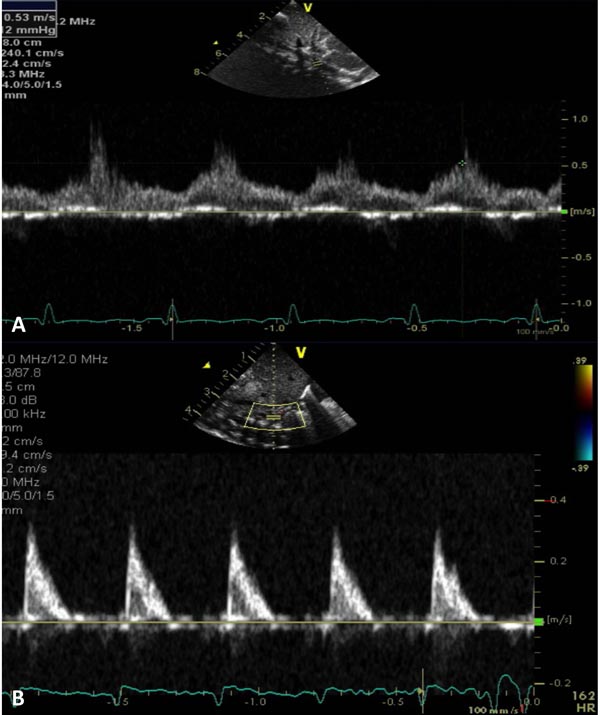
Figure 38.5. A: Pulse Doppler sampling of abdominal aorta from subcostal view shows damped trace suggestive of coarctation. B: Similar Doppler sampling of abdominal aorta in a normal neonate shows a higher, arterial type of pulsatile flow velocity with a sharp upstroke.
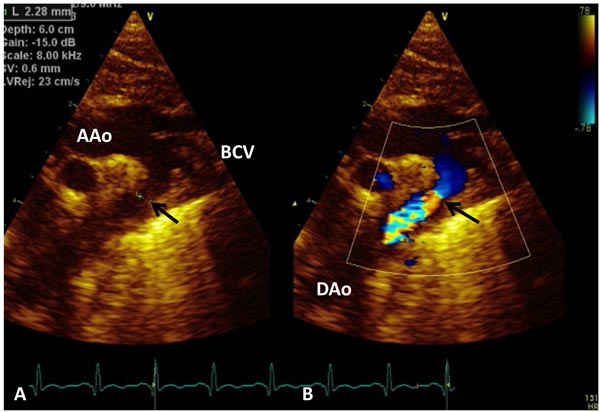
However, in the presence of a wide-open PDA, it is sometimes difficult to ensure the presence of CoA and significance of echocardiographic findings. Varying degrees of hypoplasia of the TAA and aortic isthmus are seen on echocardiographic studies (Figure 38.6).
Figure 38.6. Selected video frames from suprasternal notch views of the aortic (Ao) arch in 2D (A) and color flow (B) images of a neonate with tricuspid atresia and TGA demonstrating CoA and hypoplastic TAA.
Pulsed Doppler flow velocity proximal to site of coarctation segment is near normal (see Figure 38.4A) and increases significantly distal to the obstruction (see Figure 38.4B). Continuous wave Doppler interrogation (see Figures 38.4B and 38.7) is helpful to identify and quantify the degree of obstruction.
Figure 38.7. Continuous wave Doppler interrogation across aortic coarctation shows high velocity along with diastolic extension of the Doppler flow suggesting that the coarctation is severe.
Peak instantaneous pressure gradients across the CoA aortic are calculated by employing a modified Bernoulli equation9:
∆P = 4V2
where ∆P is peak instantaneous gradient and V is peak Doppler flow velocity in the DAo.
When there is a higher (>1.00 m/s) velocity proximal to site of coarctation, the following formula may be used to more accurately estimate the gradient9:
∆P = 4(V22 – V12)
where ∆P is peak instantaneous gradient and V2 and V1 are peak flow velocities in the descending aortic distal to coarctation (continuous wave Doppler) and proximal to the coarctation (pulsed Doppler), respectively.
Presence of pandiastolic flow (see Figures 38.4B and 38.7) or even diastolic extension of Doppler signal (see Figures 38.2D) adds to accuracy of Doppler estimation of the gradient.
Echo-Doppler studies are also very useful in defining the associated cardiac defects.
Other Imaging Studies
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree