In atherosclerosis, carotid artery stenosis (CAS), renal artery stenosis (RAS), lower extremity peripheral arterial disease (PAD), and coronary artery disease (CAD) are common pathologic lesions; their interrelationship is, however, unclear. We studied concomitant multiple atherosclerotic lesions in patients with CAD to understand their prevalence and relations. A cross-sectional analysis was performed on data from consecutive patients who underwent nonemergent coronary angiography. Simultaneous carotid and renal artery Doppler studies and ankle-brachial systolic pressure measurements were reviewed to diagnose concomitant lesions and their severity. The study included 1,734 patients (aged 71 ± 9 years; 70% men), with prevalences of CAS, RAS, lower extremity PAD, and CAD of 6%, 7%, 13%, and 72%, respectively. In patients with CAD (n = 1,253), the prevalences of CAS, RAS, and lower extremity PAD were 7%, 9%, and 16%, respectively; 24% CAD patients had ≥1 additional atherosclerotic lesion. Significant interactions among the prevalences of these lesions were found. In addition, the extent of CAD and the prevalences of CAS, RAS, and lower extremity PAD were significantly correlated. Multivariate analysis supported these relationships. In conclusion, the prevalences of CAS, RAS, lower extremity PAD, and CAD were strongly interrelated in the study population; CAD severity was related to that of other atherosclerotic lesions. Additional systematic screening of other concomitant atherosclerotic lesions is recommended, especially in CAD patients having multivessel disease, left main disease, and/or already diagnosed with other concomitant atherosclerotic lesions.
Atherosclerosis is a highly prevalent disease that greatly contributes to patient morbidity and mortality. In this study, we simultaneously analyzed the prevalences and relationships among coronary artery disease (CAD), carotid artery stenosis (CAS), renal artery stenosis (RAS), and lower extremity peripheral arterial disease (lower extremity PAD) in a large population who had undergone coronary angiography.
Methods
Between September 2010 and July 2011, 2,571 consecutive patients underwent nonemergent diagnostic catheterization on suspicion for CAD. The attending doctors decided on the need for coronary angiography based on patient symptoms and noninvasive examinations. Concomitantly, these patients were routinely screened for atherosclerotic lesions using carotid and renal artery Doppler studies and ankle brachial index (ABI) measurements, regardless of symptoms. Of these 2,571 patients, 1,734 underwent all the procedures and were eligible for inclusion in the study. Written informed consent was obtained from all patients, and ethical approval was obtained from the review committee of Shonan Kamakura (Japan) General Hospital.
Before diagnostic catheterization, demographic data, medical histories, physical examination results, and blood chemistry results were collected and entered into a computerized medical database. The following data were collected: patient’s age; gender; status with regard to diabetes mellitus (DM), hypertension (HT), dyslipidemia (DL), hyperuricemia, smoking habits, chronic kidney disease (CKD), and hemodialysis; and histories of cerebrovascular disease (CVD) and previous myocardial infarctions.
Coronary angiography was performed using standard techniques. The presence and severity of coronary atherosclerotic lesions were determined by visual estimation or by using a quantitative coronary angiography program at the discretion of the angiographer. Coronary artery lesions, graded based on the narrowing of the lumen diameter in a major epicardial artery or 1 of its major branches, were classified as significant coronary artery disease (≥75% for left anterior descending artery, left circumflex artery, right coronary artery, or ≥50% for left main trunk). Patients were stratified according to the number of involved vessels, as follows: no significant stenosis, single-vessel disease (1VD), 2-vessel disease (2VD), 3-vessel disease (3VD), and left main disease (LMD, significant stenosis of the left main trunk, with or without concomitant lesions in other vessels).
A sonography technician, blinded to clinical and coronary angiographic data, used a predefined protocol to perform internal carotid artery (ICA) duplex scanning. The degree of ICA stenosis was defined according to the current guidelines. ICA stenosis was evaluated using the Doppler-determined peak systolic velocity (PSV), peak diastolic velocity, and the maximum percentage of the diameter reduction recorded by B-mode ultrasonography. CAS severity was defined as the greatest stenosis observed on either the right or left side. Significant CAS was defined as a PSV >230 cm/s, corresponding to diameter stenosis of >70% or total or near total occlusion (defined as 0 PSV and no visible flow).
To evaluate RAS, a sonography technician, also blinded to the clinical and coronary angiographic data, used a predefined protocol to perform renal artery duplex ultrasonography. The presence and severity of RAS was evaluated by Doppler waveform analysis, using PSV, the renal/aortic ratio, and the acceleration index. RAS severity was defined as the greatest stenosis observed on either the right or left side; significant RAS was defined as a PSV >180 cm/s, corresponding to a stenosis diameter >60%. Renal artery Doppler studies were not performed in renal dialysis patients.
Lower extremity PAD was defined on the basis of ABI measurements. ABI examinations, using a continuous-wave Doppler probe, were performed by a technician blinded to each patient’s clinical and coronary angiographic data. The resting systolic blood pressure at the ankle was compared with the systolic brachial pressure, and the ratio of the 2 pressures was defined as the ABI. The severity of the lower extremity PAD was defined as the greatest stenosis observed on either the right or left side. An ABI <0.9 was diagnostic of occlusive arterial disease in patients with or without symptoms.
Continuous variables are presented as means ± SD. For univariate analysis among groups, we compared the continuous variables using Student’s 2-sample t test and categorical variables using Fisher’s exact test. Multivariate analysis was performed to identify independent correlation of the prevalence of CAS, RAS, or lower extremity PAD. Multivariate logistic regression models were used to fit the variables that were related to the prevalence of CAS, RAS, or lower extremity PAD in univariate analysis (p <0.15; the prevalence of CAD, and the extent of CAD, stratified according to the involved vessels, age, DM, HT, DL, hyperuricemia, smoking status, CKD, CVD, and history of previous myocardial infarctions). Adjusted odds ratios and 95% confidence intervals were estimated with logistic regression models. For all analyses, a 2-tailed p <0.05 was considered statistically significant. A statistical package (SPSS, Chicago, IL, USA) was used to conduct the statistical analyses.
Results
Overall, 1,734 patients (70% men) were enrolled in this study. The mean age of the patients was 71 ± 9 years; demographic and clinical data are in Table 1 . The prevalence and categorization of CAS, RAS, and lower PAD are shown in Figure 1 and listed in Table 2 . CAS, RAS, and lower extremity PAD had more concomitant risk factors for atherosclerosis.
| n (%) | |
|---|---|
| DM | 524 (30) |
| HT | 1,179 (68) |
| DL | 1,104 (63) |
| Hyperuricemia | 229 (13) |
| Smoker | 819 (47) |
| CKD | 523 (30) |
| CVD | 108 (6) |
| Previous myocardial infarction | 324 |
| Mean age (yrs) | 70 ± 9 |
| Men | 1,207 (70) |
| n (%) | |
|---|---|
| CAD | 1,253 (72) |
| CAS | 99 (6) |
| RAS | 128 (7) |
| Lower extremity PAD | 223 (13) |
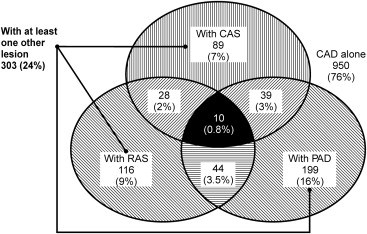
Within the population, the prevalences of CAS, RAS, lower extremity PAD, and CAD were 6%, 7%, 13%, and 72%, respectively ( Table 2 ). Among the patients with CAD (n = 1,253), 7%, 9%, and 16% of the patients were simultaneously diagnosed with CAS, RAS, or lower extremity PAD, respectively, and 24% of the patients had ≥1 of these atherosclerotic lesions ( Figure 1 ). These findings demonstrated that people diagnosed with CAS, RAS or lower extremity PAD comprised 90%, 91%, and 89% of these CAD patients, respectively. Furthermore, the presence of CAS, RAS, or lower extremity PAD was likely in CAD patients with p values <0.05 for each. In addition, 7% of the patients had at least 2 of the 3 lesions and 0.8% had all 3 lesions ( Figure 1 ).
These data indicate significant interactions among the prevalences of CAS, RAS, lower extremity PAD, and CAD. Strong correlations were found between CAS and RAS, as well as between CAS and lower extremity PAD. The prevalence of RAS in the CAS patients was 31%, and prevalence of lower extremity PAD was 43%. The prevalence of RAS increased the incidences of CAS and lower extremity PAD by 24% and 37%, respectively. Increased incidences of CAS and RAS were found in 19% and 21% of the patients with lower extremity PAD, respectively.
Significant CAS was found in 2.1%, 3.6%, 8.0%, 12%, and 10% of the patients without significant CAD, but with 1VD, 2VD, 3VD, and LMD, respectively ( Figure 2 ). Significant RAS was found in 2.5%, 3.6%, 10%, 18%, and 13% in the patients without significant CAD but having 1VD, 2VD, 3VD, and LMD, respectively ( Figure 2 ). Lower extremity PAD was found in 5.0%, 6.5%, 17%, 27%, and 30% in the patients without significant CAD, but having 1VD, 2VD, 3VD, and LMD, respectively ( Figure 2 ).
Significant relationships existed among the prevalences of CAS, RAS, and lower extremity PAD; the extent of CAD was closely related to the presence of other atherosclerotic lesions, supported by logistic regression modeling results ( Figures 3 through 5 ). Thus, RAS, lower extremity PAD, multivessel disease, and LMD were independent predictors of CAS ( Figure 3 ). Similarly CAS, lower extremity PAD, multivessel disease, and LMD were independent predictors of RAS ( Figure 4 ) and CAS, RAS, multivessel disease, and LMD were independent predictors of lower extremity PAD ( Figure 5 ). CAD, overall, was significantly associated with other atherosclerotic lesions, but the presence of 1VD was not. In addition, CVD was an independent predictor of CAS ( Figure 3 ), age was an independent predictor of RAS ( Figure 4 ), and DM, HT, smoking, CKD, and age were independent predictors of lower extremity PAD ( Figure 5 ).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


