Late gadolinium enhancement (LGE) on cardiovascular magnetic resonance imaging is associated with adverse events in adults with hypertrophic cardiomyopathy (HC). However, limited data exist on the extent and clinical significance of LGE in the pediatric population. In 30 patients (aged 14.1 ± 3.2 years) with clinically diagnosed HC who underwent cardiovascular magnetic resonance imaging from 2007 to 2012, segments with hypertrophy and LGE were identified by 2 experienced readers blinded to outcome. Radial, circumferential, and longitudinal strains were evaluated using feature tracking software. The composite outcome was defined as cardiac death, nonsustained ventricular tachycardia, ventricular fibrillation, or appropriate implantable cardioverter-defibrillator discharge. LGE was present in 17 of 30 patients (57%), all in a midmyocardial pattern, with median 3 segments per patient (interquartile range [IQR] 2 to 5). No LGE was detected in patients without phenotypic hypertrophy. Segments with LGE had decreased radial (basal segments 20.7% vs 70.9%, p = 0.01), circumferential (basal segments −23.2% vs −29.3%, p = 0.04), and longitudinal strains (basal segments −13.8% vs −20.9%, p = 0.04). After median follow-up of 26.9 months (IQR 7.5 to 34.3), 7 patients who had an adverse outcome (5 ventricular tachycardia, 1 appropriate implantable cardioverter-defibrillator discharge, and 1 death) had more segments of LGE (median 4, IQR 2 to 7 vs 0, IQR 0 to 2, p = 0.01). One patient without LGE had ventricular tachycardia on exercise test. In conclusion, LGE occurs in a similar pattern in pediatric patients with HC as in adults, associated with hypertrophy, decreased myocardial strain, and adverse clinical outcomes. Further longitudinal studies are necessary to evaluate the rate of development of LGE and relation to outcomes in a larger cohort.
Hypertrophic cardiomyopathy (HC) is a heterogeneous cardiovascular condition characterized by myocardial disarray, hypertrophy, and fibrosis, with a prevalence of 1 in 500 and varied presentations from infancy through adulthood. HC is associated with adverse outcomes, including sudden cardiac death. Myocardial fibrosis can be detected by late gadolinium enhancement (LGE) on cardiovascular magnetic resonance (CMR) image in regions of hypertrophy and decreased myocardial deformation, and has been identified in adult cohorts as a risk factor for death, ventricular tachycardia and fibrillation, appropriate implantable cardioverter-defibrillator (ICD) discharge, and unplanned cardiac admission. However, there are few data on the prevalence and clinical significance of LGE in a pediatric population, and the relation with hypertrophy and strain have not been evaluated. This study aimed to further characterize the pattern of LGE and the association of LGE with strain and clinical outcomes in pediatric patients with HC.
Methods
This single-center retrospective study included all patients aged ≤21 years with phenotypic or genotypic HC who underwent clinically indicated CMR from 2007 to 2012. HC was defined as positive genetic testing result for a mutation known to be associated with HC or clinical characteristics of asymmetric septal hypertrophy or total left ventricular mass >2 SDs above the mean for body surface area without identifiable cause. Those with coexisting congenital heart disease or an underlying syndrome or storage condition predisposing to secondary HC were excluded. This study was approved by the institutional review board.
Patient charts were reviewed for demographic data, ICD placement, and clinical outcome. A composite adverse outcome was defined as cardiac death, sustained or nonsustained ventricular tachycardia, ventricular fibrillation, or appropriate ICD discharge. Length of follow-up was defined as the time from CMR to the last clinical documentation.
CMR was performed with a 1.5-T scanner (Achieva or Ingenia; Philips, Best, the Netherlands). Cine images were obtained with breath-held, electrocardiographic-gated, segmented k-space steady-state free precession, using 30 phases per cardiac cycle. LGE images were acquired with breath-held, phase-sensitive inversion recovery in the 4-chamber and short-axis planes, 12 to 15 minutes after intravenous administration of 0.2 mmol/kg of gadoteridol (ProHance; Bracco, Monroe Township, New Jersey) or gadopentetate dimeglumine (Magnevist; Bayer, Leverkusen, Germany). Data were post-processed with QMass MR (Medis, Leiden, the Netherlands) and analyzed by 2 experienced readers blinded to patient outcome. Because of the range of patient age and size and the lack of pediatric CMR normative data for wall thickness, qualitatively hypertrophied segments were identified from short-axis images mapped on a 16-segment model and confirmed by a second reader. LGE was similarly evaluated qualitatively on a 16-segment model, enabling better matching of segments with hypertrophy and LGE.
Strain was measured with feature tracking software (TomTec, Unterschleissheim, Germany) on CMR images by manually drawing contours at the endocardial and epicardial borders, with visual evaluation to ensure adequate tracking. Basal (at the mitral valve), midventricular (at the papillary muscles), and apical (below the papillary muscles) short-axis images were analyzed for circumferential and radial strains, which are reported by level, because of previous report of a base-to-apex gradient. Two-, 3-, and 4-chamber images were used for longitudinal strain.
Data are presented as frequency (percent) for categorical variables and mean ± SD or median (interquartile range [IQR]), as appropriate, for continuous variables. Association of LGE and hypertrophy was evaluated by generalized estimating equation to account for correlation across segments within patients. Similarly, repeated-measures analysis of variance was used to compare strain in segments with LGE versus segments without LGE and in segments with hypertrophy and LGE versus segments with hypertrophy alone. Clinical outcomes were compared between the patients with and without LGE using chi-square test or Fisher’s exact test for categorical variables and Wilcoxon rank sum test for continuous variables. Odds ratio (OR) and 95% confidence interval (CI) of having LGE for each outcome are reported. All analyses were performed using SAS, version 9.3 (SAS Institute Inc., Cary, North Carolina), with statistical significance set at a p value of <0.05 using 2-sided test.
Results
Of 51 patients screened for possible HC, 30 were included in the study population (aged 14.1 ± 3.2 years), with individual data given in Table 1 . Of those excluded, 2 had associated congenital heart disease, 1 had secondary HC associated with a syndrome, and 18 lacked adequate phenotypic or genotypic evidence of HC. In this cohort, 2 patients were siblings, 11 of 12 patients with genetic testing had mutations associated with HC, and 6 patients were genotype positive without phenotypic evidence of hypertrophy. This cohort was largely asymptomatic, with no patients in clinical heart failure, and all patients had normal left ventricular systolic function (left ventricular ejection fraction 65.4 ± 8.3%).
| Age (yrs) | Gender | BSA (m 2 ) | Genotype | Myectomy | LVOTO | NYHA | LVEDVi | LVESVi | LVMi | LGE | ICD | End Point |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 7.0 | M | 0.98 | Yes | Yes | I | 97 | 31.6 | 134 | 2 | Yes | No | |
| 7.7 | F | 1.08 | No | No | I | 57 | 13.0 | 72 | 0 | No | No | |
| 8.9 | F | 1.6 | Yes | Yes | II | 72 | 11.4 | 164 | 3 | Yes | No | |
| 9.4 | M | 1.9 | Yes | Yes | II | 118 | 25.8 | 125 | 4 | Yes | No | |
| 10.3 | F | 1.24 | MYBPC3 | No | No | I | 77 | 29.0 | 37 | 0 | No | No |
| 10.6 | M | 1.5 | MYH7 | No | No | I | 95 | 27.3 | 45 | 1 | Yes | VT |
| 11.9 | M | 1.23 | No | No | I | 65 | 21.1 | 44 | 0 | No | No | |
| 12.0 | M | 1.77 | No | No | I | 85 | 22.6 | 181 | 5 | Yes | VF | |
| 12.1 | M | 1.37 | MYH7 | Yes | Yes | I | 88 | 30.6 | 98 | 2 | Yes | No |
| 12.8 | M | 1.9 | No | Yes | I | 112 | 30.5 | 159 | 1 | No | No | |
| 12.8 | F | 1.46 | No | No | I | 72 | 24.6 | 84 | 3 | Yes | VT | |
| 13.9 | F | 1.38 | MYBPC3 | No | No | I | 85 | 31.1 | 45 | 0 | No | No |
| 14.2 | M | 1.81 | No | Yes | I | 92 | 27.6 | 101 | 0 | No | No | |
| 14.5 | F | 1.85 | MYBPC3 | No | No | I | 77 | 33.0 | 56 | 0 | No | No |
| 14.8 | M | 2.03 | No | No | I | 134 | 47.8 | 158 | 7 | Yes | VT | |
| 14.9 | F | 1.78 | No | No | I | 106 | 38.8 | 83 | 2 | Yes | No | |
| 14.9 | F | 1.6 | MYH7 | No | I | 82 | 25.6 | 47 | 0 | Yes | No | |
| 15.4 | M | 1.93 | Negative | No | No | II | 108 | 40.4 | 86 | 3 | No | Death |
| 15.5 | F | 1.91 | No | Yes | II | 78 | 24.1 | 137 | 4 | Yes | VT | |
| 16.0 | M | 1.98 | MYBPC3 | No | No | I | 92 | 28.3 | 53 | 0 | No | No |
| 16.1 | F | 1.52 | MYBPC3 | No | No | I | 84 | 27.0 | 65 | 0 | No | No |
| 16.8 | M | 1.73 | MYBPC3 | No | No | I | 102 | 43.3 | 64 | 0 | Yes | No |
| 17.0 | M | 1.78 | MYH7 | No | No | I | 87 | 28.6 | 62 | 2 | No | No |
| 17.2 | F | 1.8 | No | No | I | 83 | 27.2 | 51 | 0 | No | No | |
| 17.2 | F | 1.93 | No | No | I | 69 | 28.0 | 73 | 3 | No | No | |
| 17.5 | M | 2.16 | MYBC3 | No | No | I | 90 | 40.7 | 67 | 0 | No | No |
| 17.7 | M | 1.83 | No | No | I | 92 | 24.0 | 79 | 2 | No | No | |
| 17.7 | F | 1.53 | No | Yes | I | 84 | 25.5 | 102 | 8 | No | No | |
| 18.1 | M | 1.76 | No | No | I | 96 | 34.6 | 77 | 0 | Yes | VT | |
| 19.3 | M | 1.98 | No | No | I | 88 | 39.9 | 91 | 0 | No | No |
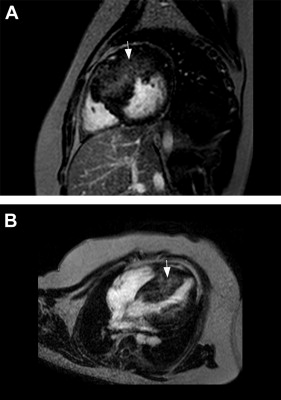
Hypertrophy was identified in at least 1 segment in 24 patients (80%), with a median of 5 segments per patient (IQR 2 to 6). LGE was present in 17 patients (57% of the cohort; 71% of patients with phenotypic HC) with a median of 3 segments per patient (IQR 2 to 5). All LGE occurred in a midmyocardial pattern ( Figure 1 ), most prevalent in the basal, midanterior and inferior septal segments ( Figure 2 ). LGE was highly associated with hypertrophy; segments with LGE had an OR of 38.6 for being hypertrophied (95% CI 14.3 to 101, p <0.0001). No LGE was identified in patients without phenotypic hypertrophy.