Chapter 30 Clinical Presentation and Diagnosis of Cerebrovascular Disease
Stroke is a common and serious disorder. Each year stroke affects almost 800,000 people in the United States and about 16 million people throughout the world.1 Associated high morbidity and mortality provide impetus for improving diagnosis, acute management, and prevention of strokes. A full understanding of how patients with stroke and cerebrovascular disease come to medical attention, along with a logical approach for defining the mechanism of stroke, are needed for safe and effective implementation of acute therapies and prevention strategies. This chapter will focus on clinical manifestations of all types of cerebrovascular disease and how clinicians can approach diagnostic evaluation.
Overview of Clinical Stroke
Stroke and cerebrovascular disease are caused by some disturbance of the cerebral vessels in almost all cases. In simple terms, we can divide stroke into two major types: ischemic and hemorrhagic. Ischemic stroke is the most common variety and is responsible for 80% to 85% of all strokes; hemorrhagic stroke accounts for the remainder.2 On occasion, an ischemic stroke can undergo secondary hemorrhagic transformation; likewise, a cerebral hemorrhage (particularly a subarachnoid hemorrhage [SAH]) can cause a secondary ischemic stroke via vasospasm.
Clinical Manifestations of Stroke and Cerebrovascular Disease
Stroke is similar to real estate in that much of its presentation and prognosis depend on size and location. The area of brain involved dictates presenting symptoms. Blood vessels that supply different parts of the brain are affected by different types of cerebrovascular disease and have different mechanisms (pathophysiology) for the stroke. This concept greatly influences and defines the approach a vascular neurologist or neurosurgeon uses when assessing patients with a stroke or cerebrovascular disease.3,4
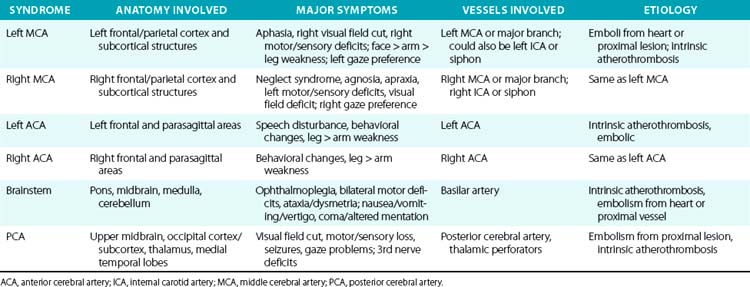
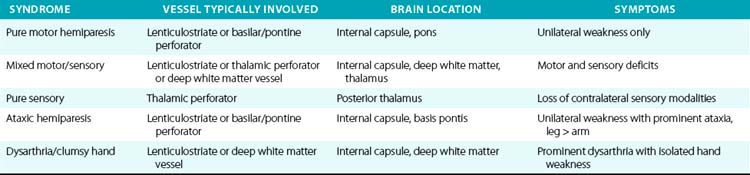
For example, a patient with evidence of involvement of the left hemispheric cortex (e.g., aphasia, visual field defect, weakness of contralateral face and arm) is likely to have a process involving the left middle cerebral artery (MCA). If head computed tomography (CT) does not show evidence of a hemorrhage, likely etiologies would include an embolic event from the heart (e.g., atrial fibrillation) or an artery-to-artery embolism (as might be seen with a high-grade lesion at the carotid bifurcation in the neck). Another patient with a pure motor hemiparesis but no other deficits is likely to have a lesion affecting the motor pathways in the internal capsule, often due to occlusion of a small penetrating artery (lenticulostriate vessel) deep in the brain. Most ischemic strokes will respect the vascular territory of one or more arteries.5 Indeed, lesions that do not respect typical arterial territories lead to concern for a nonvascular process (e.g., tumor, infection). Common ischemic stroke syndromes can be found in Tables 30-1 and 30-2.
Similar reasoning holds true for most cases of hemorrhagic stroke. Intracerebral hemorrhage often presents with abrupt onset of symptoms, but close questioning may reveal that symptoms actually progressed over 15 to 30 minutes as the hematoma grew and expanded.6 Subarachnoid hemorrhage is often characterized by sudden onset of the worst headache of one’s life, with significant nausea, vomiting, and stiff neck in many cases. The phrase “worst headache of my life” is so characteristic of SAH that a patient who presents to the physician or emergency department with that symptom complex is assumed to have SAH until proven otherwise.7
Transient Ischemic Attack
A transient ischemic attack (TIA) is often a prodrome to an ischemic stroke. Symptoms of a TIA are identical to those of a stroke, but with resolution within 24 hours (according to the old definition of a TIA). In reality, most TIA syndromes last just a few minutes, not many hours. In fact, modern brain imaging using magnetic resonance imaging (MRI) with diffusion-weighted sequences has now shown that 25% to 30% of patients with a TIA lasting 30 min to 2 hours will have a new diffusion-weighted imaging (DWI) lesion on MRI indicating a stroke based on a tissue definition.8 Transient ischemic attack symptoms lasting 6 hours or longer have a 50% likelihood of having a new stroke on MRI with DWI techniques. Therefore the perceived distinction between a TIA and a stroke should be viewed more as a continuum from minor transient neuronal dysfunction to permanent brain infarction.
Although it was once thought that the risk of stroke after a TIA was low, new imaging studies as well as epidemiological studies have proven this is not the case. Based on purely clinical criteria (not MRI results), several recent studies have shown that after a TIA, 10% of patients will have a stroke within 3 months, and half those strokes (5%) will occur within 48 hours of the initial TIA. About 25% of patients with a TIA will have a stroke, myocardial infarction (MI), death, recurrent TIA, or be hospitalized within the next 3 months.9 Based on these poor outcomes, recently published guidelines recommend hospital admission for patients with a recent TIA.8
Further studies have attempted to better define those patients with a TIA who are at higher risk of having a stroke within the next 2 to 7 days. Several scoring systems have been developed (Table 30-3) that may be useful for assessing such risks. Of course, any such assessment tool must be tempered by good clinical judgment and consideration of all clinical factors.
Table 30-3 Transient Ischemic Attack Scoring Systems
| ABCD | Age, blood pressure, clinical symptoms, duration |
| ABCD2 | Age, blood pressure, clinical symptoms, duration, diabetes |
| ABCD2I | Age, blood pressure, clinical symptoms, duration, infarction |
| Age: 60 years or greater = 1 point Blood pressure: systolic 140 mmHg or greater = 1 point or diastolic 90 mmHg or greater = 1 point Clinical symptoms: unilateral weakness = 2 points; speech disturbance without weakness = 1 point Duration: 60 minutes or more = 2 points; 10-59 minutes = 1 point Diabetes: 1 point (on antidiabetic medications) Infarction: evidence for acute ischemic stroke on CT or MRI | |
CT, computed tomography; MRI, magnetic resonance imaging.
From Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al: Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 369:283–292, 2007.50
Several types of TIAs deserve special mention because of their unique presentations. One is sudden blindness in one eye, which typically occurs as a “shade coming down” over the eye. Some patients report a graying out of vision in the eye, like looking through a gray haze or cloud. This type of TIA is often referred to as amaurosis fugax. This symptom complex typically resolves in a few minutes, although it can last for several hours. There is sometimes pain in or around the eye, but patients usually do not have any other focal neurological complaints. Some cases of amaurosis are due to emboli to the retinal circulation from an ulcerated plaque in or near the carotid bifurcation in the neck. Other cases can be due to local disease in the ophthalmic artery or in the posterior ciliary artery that supplies the optic nerve.10
The other unique type of TIA is the limb-shaking TIA. This typically involves the arm or leg on one side of the body. Patients report uncontrollable shaking of a limb that can be precipitated by movement. These spells can last seconds to minutes. They are not epileptic in origin; electroencephalogram (EEG) is unremarkable. These TIAs are associated with severe stenosis of the contralateral internal or common carotid artery.11 Once the carotid artery is opened (usually with an endarterectomy), the spells cease.
Lastly is the topic of crescendo TIAs. This refers to a pattern where TIAs are recurrent, last longer, or are more severe in nature. This is a very worrisome type of TIA and is associated with a risk of stroke as high as 25% to 50% over the next few weeks.12
Some hemorrhagic strokes may also have a TIA equivalent, namely the sentinel headache before a SAH. The sentinel headache present as an acute headache that is unusual in terms of its nature, severity, and onset. It typically lasts more than an hour but does not have other impressive focal neurological findings and resolves prior to the definitive SAH presentation. Sentinel headaches occur in 25% to 50% of patients with a subsequent aneurysmal SAH and typically antedate the SAH by days to weeks (average 2 weeks).13,14 It is thought that most of these headaches are due to either minor leakage from a fragile aneurysm or enlargement of the aneurysm, resulting in pressure on a nearby structure that produces pain.
Ischemic Stroke Syndromes
There are numerous manifestations of ischemic stroke, and they can be classified based on brain location involved, artery affected, or symptoms produced. Although advanced diagnostic techniques have altered some of the clinical rules of stroke symptoms and etiology, there are still some useful concepts that can guide us in terms of stroke location and mechanism. Tables 30-1 and 30-2 list some classic ischemic stroke syndromes with their major clinical manifestations, vascular territory, and underlying pathophysiology.5
Broadly speaking, ischemic strokes typically involve one or more vessels or vascular territories and produce a clinical picture of focal neurological deficit. Typically, clinicians look for unilateral weakness or sensory deficits, unilateral visual field abnormalities, speech disturbance (aphasia or dysarthria), neglect syndromes, unilateral ataxia, ophthalmoplegias, or gaze abnormalities as clues of a stroke. Symptoms such as vague diffuse weakness alone, headaches alone, memory loss, abnormal behavior, or isolated dizziness are rarely caused by an ischemic stroke. The appearance of a lesion in a typical vascular territory (based on brain imaging) is a key feature of almost all stroke syndromes.4
Presence of cortical deficits (aphasia, visual field cuts, neglect syndromes) often indicates involvement of a major cerebral vessel in the cerebral hemispheres. Presence of ataxia, bilateral motor or sensory deficits, Horner’s syndrome, ophthalmoplegias, and crossed sensory findings (one side of the face and the other side of the body) often indicates a stroke in the posterior (vertebral-basilar) territory. There are specific syndromes that indicate small-vessel involvement deep in the brain. These so-called lacunar strokes are due to occlusion of small penetrating arteries that arise directly from larger parent vessels. Favored locations include the deep basal ganglia structures, thalamus, and brainstem (pons). A listing of large-vessel and lacunar syndromes appears in Tables 30-1 and 30-2.
Atherothrombosis accounts for the majority of ischemic strokes. These lesions can occur anywhere in the cerebral vasculature, but they tend to have a preference for specific locations such as the bifurcation of the carotid artery in the neck, intracranial carotid siphons, proximal portion of the middle cerebral artery, mid-portion of the basilar artery, and aortic arch. An atherosclerotic plaque forms over many years, then ruptures causing formation of a superimposed thrombus.15 This atherothrombotic lesion can totally occlude the vessel, produce severe narrowing (leading to watershed ischemia), or be a source of embolic material that embolizes to more distal parts of the cerebral vasculature (artery-to-artery emboli).
Cardiac embolism accounts for 15% to 20% of all ischemic strokes. A variety of conditions such as atrial fibrillation, endocarditis, prior myocardial infarction, valvular disease, and cardiomyopathy often lead to formation of intracardiac thrombi that subsequently embolize to the brain (and other organs).4,16 Most lacunar strokes are due to either lipohyalinosis or microatheromata occluding a small penetrating artery.
Special Cases
Ischemic Stroke in Young Adults
All clinicians see young adults (often defined as ≤ 45 years of age) with ischemic strokes. Such cases often entail a special evaluation because of the unique processes and conditions that can produce strokes in this age group. Many case series have examined the diseases leading to ischemic strokes in the young, and in general they fall into a few major categories: (1) premature atherosclerosis, (2) unusual vascular pathologies, (3) cardiac etiology, (4) coagulopathy, and (5) other diseases.17
Premature atherosclerosis typically occurs in patients with risk factors for atherosclerosis; in some cases these have not been diagnosed or not properly treated. Examples include hypertension, hyperlipidemia, diabetes, smoking, and obesity. The types of uncommon vascular pathologies often seen in young adults with a stroke include dissection of a vessel (often not related to any obvious trauma), fibromuscular dysplasia, moyamoya disease, or a vasculitis related to an inflammatory condition or drug abuse.17 Numerous cardiac processes can lead to strokes in the young, such as congenital heart disease, a patent foramen ovale (particularly with evidence of venous thrombi), valvular disease (infectious or inflammatory), cardiomyopathy, myxoma, papillary fibroelastoma, and many others. Myriad clotting disorders have been associated with strokes in young adults, the most common being lupus anticoagulants, anticardiolipin antibodies, and protein C and protein S deficiency.18 In general, these coagulopathies are more likely to cause venous thrombosis than arterial thrombosis. Clotting disorders related to hematological malignancies can cause both ischemic and hemorrhagic strokes.19 Various systemic diseases are also associated with hypercoagulable states such as inflammatory bowel disease, hemoglobinopathies, elevated homocysteine, and cancer.
The “other” category covers a host of conditions, some rare and some common, that cause strokes in young adults. Migraine headaches and pregnancy are the most common of these. Patients with complex or complicated migraines, prolonged auras, or taking contraceptives have a higher risk of stroke.20 Pregnancy, particularly in the third trimester and up to 3 months postpartum is associated with increased stroke risk, particularly venous thrombosis and cerebral hemorrhage.21,22 Drug abuse is another common cause of ischemic and hemorrhagic strokes in young adults.23 Other rare conditions include CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy), MELAS (mitochondrial encephalomyopathy, lactic acidosis, and stroke), isolated central nervous system (CNS) vasculitis, Sneddon syndrome (combination of a livedo reticular rash, antiphospholipid antibodies, and ischemic stroke), Marfan’s syndrome, and a host of others (especially connective tissue disorders) have been known to cause strokes in this population.
Strokes Related to Systemic Disease
There are a number of unique systemic disorders that cause strokes in patients of any age. Autoimmune diseases, such as lupus, can produce strokes through a variety of mechanisms that include advanced or premature atherosclerosis, vasculitis, hypercoagulable states, and cardioembolic events.24 Sickle cell disease (SCD) also leads to ischemic strokes and hemorrhagic strokes due to myriad processes including a large-vessel arteriopathy, small-vessel occlusion, rupture of moyamoya vessels (producing ICH and/or SAH), and accelerated atherosclerosis due to hypertension and renal failure.25,26
Drug abuse, particularly cocaine, can produce ischemic strokes via a number of processes including vasospasm, cardiac emboli (due to cardiomyopathy), hypertension, and endocarditis.27 Drug abuse can produce an ICH or SAH due to extreme hypertension and necrotizing vasculitis. It is a fallacy to assume that drug abuse only occurs in young patients or those from certain demographic groups. All patients admitted with a stroke should be tested for drug abuse with urine toxicology screens, not excluding those older than 50 years and white-collar professionals.
Human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) is now recognized as increasing the risk of stroke. This is partially because patients with HIV/AIDS are living longer, and some are having strokes as a result of accelerated development of typical stroke risk factors. It is also clear that modern drug therapy for AIDS can increase the risk of stroke (particularly ischemic stroke).28,29
Systemic cancer is a commonly overlooked cause of strokes. Sometimes the stroke diagnosis precedes diagnosis of the underlying cancer. Mechanisms for strokes related to cancer include a hypercoagulable state and nonbacterial thrombotic endocarditis. Oftentimes these strokes are multiple, variable in size, and in different vascular territories.30,31 Such patients may also have deep venous thrombosis (DVT). Liver failure appears to increase risk of ischemic and hemorrhagic stroke.
Intracerebral Hemorrhage
Chronic or acute hypertension is the most common etiology for nontraumatic ICH, and this type of bleed typically occurs in specific brain locations (Table 30-4). As with ischemic stroke, location of the ICH is highly correlated with the type of symptoms produced. Recent studies using serial brain scans have shown that 30% to 40% of ICHs will expand over the first 24 hours after admission; such expansion is almost always associated with clinical worsening.6,32 High blood pressure may be a risk factor for ICH expansion, although this association has not been mechanistically proven.
Table 30-4 Location and Symptoms for Common Types of Intracerebral Hemorrhage
| ICH Location | Likely Etiology | Common Symptoms |
|---|---|---|
| Basal ganglia | Hypertension | Contralateral hemiparesis, speech changes, gaze deviation, altered mentation if large |
| Lobar | Hypertension, CAA | Cortical syndromes, weakness, visual field lesions, altered mentation if large |
| Thalamus | Hypertension | Altered mentation, sensory changes, gaze abnormalities |
| Pons | Hypertension | Coma, gaze and pupil abnormalities, quadriparesis |
| Cerebellum | Hypertension, AVM | Ipsilateral ataxia, dizziness, vertigo, nausea/vomiting |
| Hemispheric cortex | AVM, extreme hypertension, mycotic aneurysm | Headaches, seizures, cortical syndromes |
AVM, arteriovenous malformation; CAA, cerebral amyloid angiopathy; ICH, intracerebral hemorrhage.
Another increasingly common etiology for ICH is cerebral amyloid angiopathy (CAA), which typically affects patients older than 70 years of age. Cerebral amyloid angiopathy is caused by deposition of one or more amyloid proteins within the wall of cerebral small arterioles. A typical CAA bleed occurs in a lobar region (junction of gray matter and white matter), most commonly in the parietal, temporal, and occipital lobes. Intracerebral hemorrhages due to CAA can be multiple and recurrent.33–35 There is a clear association between CAA, ICH, and Alzhemier’s disease. Sometimes an ischemic stroke can undergo hemorrhagic transformation and become an ICH. This occurs in up to 15% of cases of ischemic stroke and is associated with large strokes, cardioembolic strokes, and the use of anticoagulants and thrombolytic agents.
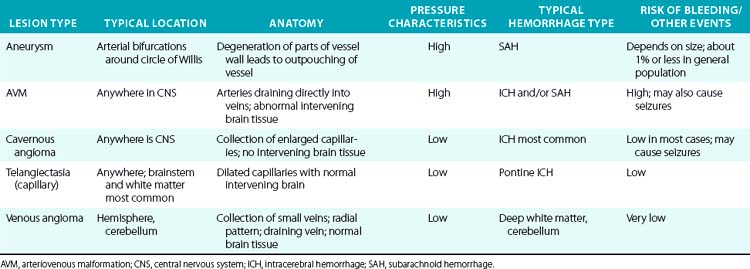
A variety of vascular malformations can cause an ICH, particularly arteriovenous malformations (AVMs) and cavernous malformations (less commonly, capillary telangiectasias and developmental venous anomalies). Arteriovenous malformations are the most common and serious type of vascular malformation that cause an ICH, and recurrent ICHs, as well as producing seizures and local neurological deficits.36 The characteristics and hemorrhagic risk of each of these lesions is shown in Table 30-5.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree