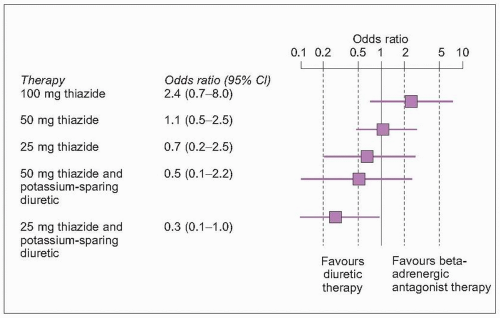
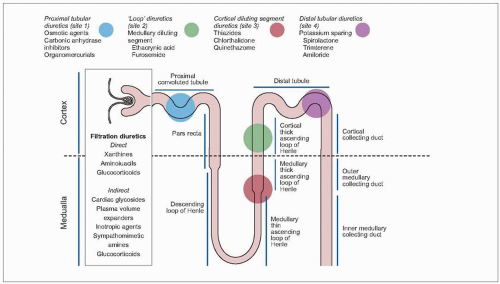
Bumetanide, ethacrynic acid and furosemide are the most potent loop-acting diuretic agents in clinical use. Unlike the thiazides, they promote natriuresis by inhibiting sodium transport at the ascending limb of the loop of Henle (
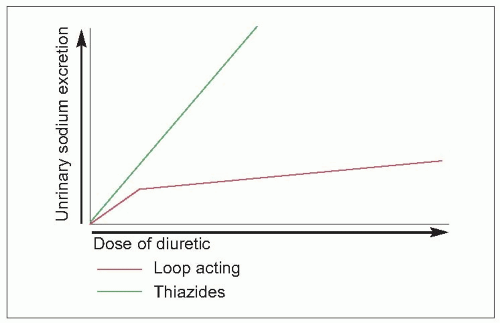
3.1). Since more of the filtered sodium is delivered to the distal tubule for exchange, a greater degree of potassium wastage results. Their onset of action is more immediate, frequently within 20 minutes. Consequently, the diuresis is more rapid than the thiazides and their congeners produce, the rebound sodium and water retention may be more pronounced, and there may be greater potassium wastage. For these reasons, these compounds should be reserved for patients who cannot take the thiazides or when more prompt diuresis is desired, in patients with renal functional impairment or when an intravenous diuretic is necessary. In those patients with renal functional impairment, the dose-response curve of the loop agents is linear, unlike that of the thiazides (
3.2). Thus, the ‘loop diuretics’ are not recommended for patients with uncomplicated, essential hypertension. In patients with secondary hyperaldosteronism (e.g. congestive heart failure), particular care should be exercised to prevent hypokalaemia and cardiac dysrhythmias. These patients present a very real potential for sudden cardiac death (
3.4). One final important, but occasionally overlooked, indication for these agents is in the hypertensive patient who is already receiving antihypertensive drugs, including maximal thiazide doses. These patients may have developed pseudotolerance as a consequence of intravascular volume expansion, and this may be overcome by switching to the more potent loop-acting agent.