Chapter 23 Clinical Evaluation of Renal Artery Disease
Data from the National Health and Nutrition Examination Survey (NHANES) 2005 to 2008, extrapolated to 2008, estimated that approximately 76,400,000 adults 20 years of age or older have essential (primary) hypertension.1 Renovascular disease and renal parenchymal disease are the most common secondary causes of hypertension after obesity, excess alcohol ingestion, drug abuse, and oral contraceptive use are excluded.
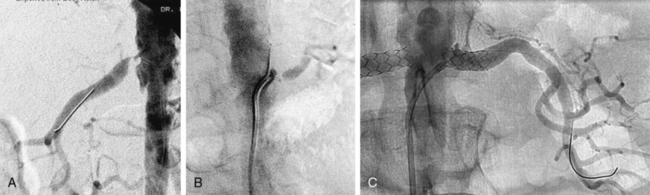
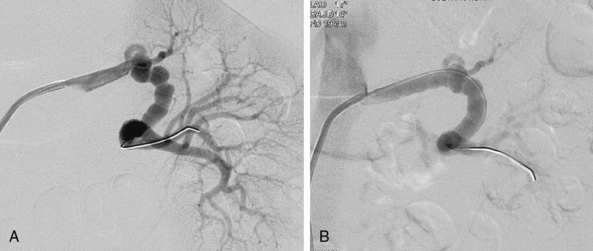
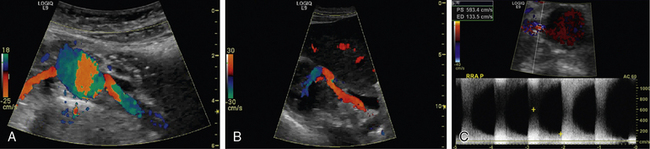
The presence of anatomical renal artery stenosis (RAS) does not necessarily establish that the hypertension or renal failure is due to RAS. Incidentally discovered RAS is quite common, whereas renovascular hypertension only occurs in 1% to 5% of all patients with hypertension.2,3 Approximately 90% of all renovascular disease is caused by atherosclerosis.4,5 Fibromuscular dysplasia (FMD) is the second most common cause of RAS.6 Patients with atherosclerotic RAS are typically older than age 55 and have the usual risk factors for atherosclerosis, but FMD is more common in younger women. The predominant clinical manifestation of FMD is hypertension; atherosclerotic RAS may present with hypertension, renal failure (ischemic nephropathy), and/or recurrent episodes of congestive heart failure (CHF) and “flash” pulmonary edema.7 Whereas atherosclerotic RAS most often occurs at the ostium or proximal portion of the renal artery, FMD usually occurs in the mid- to distal renal artery and its primary branches (Figs. 23-1 and 23-2).
The effects of atherosclerosis on the coronary and carotid arteries are well recognized, but involvement of the renal arteries is frequently overlooked. In addition to the sequelae of RAS (hypertension, renal failure), patients with atherosclerotic RAS succumb prematurely from myocardial infarction (MI) and stroke.8–11 Thus, early diagnosis and treatment is important to avoid the consequences of RAS.
When considering the diagnosis of RAS, it is useful to think in terms of the circumstances in which RAS is likely to occur (Box 23-1).
![]() Box 23-1 Clinical Clues That Suggest Presence of Renal Artery Stenosis
Box 23-1 Clinical Clues That Suggest Presence of Renal Artery Stenosis
Hypertension
Individuals who develop hypertension between the ages of 30 and 55 usually have primary (essential) hypertension. If the initial diagnosis of hypertension is made before the age of 30, it is usually due to FMD if other known secondary causes (obesity, oral contraceptive use, drug abuse, and parenchymal renal disease) have been excluded. Since atherosclerosis occurs in older individuals, it is usually the cause of RAS after the age of 55. In one population-based study of Medicare patients aged 65 or older, the prevalence of atherosclerotic RAS was 6.8%.12 In this cohort, RAS was found in nearly twice as many men as women (9.1% vs. 5.5%; P = 0.053); no significant differences were identified between Caucasians and African American subjects (6.9% vs. 6.7%; P = 0.933).12 Although RAS can be associated with both systolic and diastolic hypertension, the diagnosis of RAS should be seriously considered in individuals who present with new-onset diastolic hypertension after the age of 55, primarily because diastolic blood pressure usually declines after age 55 in normal individuals. It is not uncommon for patients to have primary hypertension for many years, and as they age, develop atherosclerotic RAS. This cohort of patients may have had well-controlled blood pressure that suddenly becomes more difficult to control.
Patients may have anatomically significant RAS and no hypertension at all. Dustan and colleagues reviewed 149 aortograms and found that approximately half of patients with 50% or more RAS did not have hypertension.13 Moreover, in a recent systematic review of 40 studies that evaluated a total of 15,879 patients, the mean prevalence of RAS among patients with suspected renovascular hypertension was 14.1%.11 On further analysis of the patients who were incidentally found to have RAS on imaging studies, 65.5% were hypertensive and 27.5% had renal failure.11 Therefore, the mere presence of RAS and hypertension does not necessarily mean that one is causing the other.
Accelerated or malignant hypertension also has been associated with a very high prevalence of RAS.14Resistant hypertension is defined as failure to normalize blood pressure to less than 140/90 mmHg following an optimal medical regimen consisting of at least three drugs with different mechanisms of action, including a diuretic.15 The diagnosis of renovascular disease should be strongly considered in patients with true drug-resistant hypertension.
Renal Abnormalities
Gifford et al. found that 71% of patients (53 of 75 patients) with an atrophic kidney had severe stenosis or complete occlusion of the renal artery supplying the small kidney.16 Three studies have shown that if there is a discrepancy in size between the two kidneys or if one kidney is atrophic, the contralateral renal artery (normal-sized kidney) is severely stenotic about 60% of the time.2,16,17 Therefore, the presence of an atrophic kidney or a discrepancy in size between the two kidneys demands a thorough investigation for the presence of renovascular disease.
Numerous reports suggest that patients who develop azotemia while receiving angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blocking (ARB) agents have bilateral RAS, RAS to a solitary functioning kidney, or decompensated CHF in the sodium-depleted state.18–22 These clinical scenarios are absolute indications for investigation, since they usually reflect the presence of severe RAS to the entire functioning renal mass, thus placing the patient in jeopardy of renal failure. The mechanisms of acute and chronic renal failure in patients with RAS are discussed in detail in Chapter 22.
There are no prospective studies evaluating how often atherosclerotic renovascular disease leads to end-stage renal disease (ESRD). Scoble et al. found that atherosclerotic renovascular disease was the cause of ESRD in 14% of patients starting dialysis therapy.23,24 In a retrospective review over a 20-year period in 683 patients, 83 (12%) patients had documented RAS as a cause of ESRD. Since arteriography was only performed in patients with suspected RAS, it is entirely possible that the true incidence of RAS as a cause of ESRD was underestimated. De Mast and Beutler reported that 41% of patients with ESRD had at least one renal artery with more than 50% stenosis.11 Renal artery stenosis must be excluded in every patient starting dialysis if a clear-cut etiology for the ESRD is not known because the mortality in this patient population is extremely high. In the series by Mailloux et al., median survival in patients with ESRD secondary to RAS was 25 months, while 2-, 5-, and 10-year survival was 56%, 18%, and 5%, respectively.9,10
Effects of Renal Artery Stenosis on the Heart
Recurrent CHF and flash pulmonary edema unrelated to ischemic heart disease can result from bilateral RAS (or unilateral RAS to a single functioning kidney). In one renal artery stent series, 39 patients (19% of all patients undergoing renal artery stent implantation from 1991–1997) had recurrent episodes of CHF or flash pulmonary edema as the primary indication for renal artery stenting.25 Nineteen of 39 patients had moderate to severe left ventricular (LV) systolic function. Although not completely understood, the mechanism of CHF may be related in part to the inability to use ACE inhibitors or ARBs to the direct adverse effects of angiotensin II (Ang II) on myocardial function, or to the inability to control volume adequately. If coronary ischemia has been excluded as a cause of CHF, renal revascularization (percutaneous stenting or surgical) is a very effective method of treatment in these individuals.25–27
One retrospective study demonstrated improvement in anginal symptoms in patients undergoing renal artery stent implantation. The mechanism of such improvement is not clearly delineated, but 88% of these patients had improved blood pressure control after stenting. This effect may account at least in part for decreased anginal symptoms.28
Presence of Atherosclerosis in Other Vascular Beds
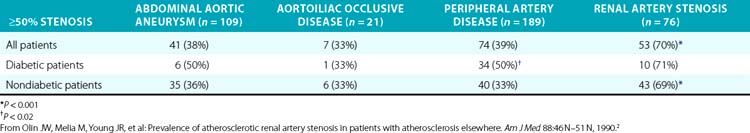
Several series have examined the prevalence of renovascular disease in patients who have atherosclerotic disease elsewhere. To determine the prevalence of atherosclerotic RAS, Olin et al. studied 395 consecutive patients who had undergone arteriography as part of an evaluation for either an abdominal aortic aneurysm, aortoiliac occlusive disease, or peripheral artery disease (PAD)2 (Table 23-1). These patients did not have the usual clinical clues to suggest RAS. High-grade bilateral renal artery disease was present in approximately 13% of patients. In addition, 76 patients had an aortogram performed for suspected RAS, and RAS was present in 70% of these subjects. Other studies have shown that 22% to 59% of patients with PAD have significant RAS.29
It has also been established that RAS is common in patients with coronary artery disease (CAD). Of 7758 patients undergoing cardiac catheterization during a 78-month period of time, 3987 underwent aortography at the time of catheterization to screen for RAS30; 191 (4.8%) had more than 75% RAS, and 0.8% had severe bilateral disease. In a Mayo Clinic series, renal arteries were studied at the time of cardiac catheterization in patients with hypertension.31 Ninety percent of the renal arteries were adequately visualized, and no complications occurred from the aortogram. More than 50% RAS was present in 19.2%, more than 70% stenosis in 7%, and bilateral RAS was present in 3.7% of patients. The likelihood of significant RAS is markedly increased in patients with two or more coronary artery lesions.32 A prospective study of the long-term natural history of patients undergoing cardiac catheterization and renal angiography is needed to determine whether diagnosing RAS in this setting improves patient outcome measures. Renal artery disease is also associated with atherosclerotic disease in the carotid arteries. Louie et al. demonstrated that 46% of patients with more than 60% RAS also had more than 50% carotid stenosis.33 All of these studies support the fact that atherosclerotic RAS is a manifestation of systemic atherosclerosis and reinforce the concept of treating the entire patient, not just the circulatory bed involved at a given point in time.
The presence of RAS even prior to development of ESRD portends a poor prognosis. Patient survival decreases as the severity of RAS increases, with 2-year survival rates of 96% in patients with unilateral RAS, 74% in patients with bilateral RAS, and 47% in patients with stenosis or occlusion to a solitary functioning kidney.34 Dorros et al. demonstrated that as serum creatinine increases, survival decreases in patients with atherosclerotic RAS.35 The 3-year probability of survival was 92 ± 4% for patients with a serum creatinine below 1.4 mg/dL, 74 ± 8% for patients with a serum creatinine of 1.5 to 1.9 mg/dL, and 51 ± 8% for patients with a serum creatinine 2.0 mg/dL or higher.
Long-term survival was investigated in a cohort of 1235 patients who underwent abdominal aortography at the time of cardiac catheterization. The 4-year survival rate of patients without RAS was 88% versus 57% for those with RAS.34
Physical Examination
The physical examination is generally not helpful in the diagnosis of RAS. Evidence of coronary, cerebral, or PAD is associated with a higher likelihood of renal artery disease because of the systemic nature of atherosclerosis. A systolic abdominal bruit is common and nonspecific, but the presence of both a systolic and diastolic bruit auscultated over the epigastrium may point to underlying renal artery disease.36 Presence of a diastolic component to the bruit indicates that the degree of narrowing of the artery is severe, since there is continued flow during diastole.37 An abdominal bruit with a systolic and diastolic component occurs more often in patients with FMD (53%) than in patients who have atherosclerotic disease (12.5%).36 Presence of a bruit is helpful, but absence does not exclude the diagnosis of either atherosclerotic renovascular disease or FMD.
Diagnosis of Renovascular Disease
In the past, indirect methods of assessing the renal arteries were commonly used to diagnose RAS. Intravenous urography is obsolete as a screening tool, owing to its poor sensitivity and specificity.38 Plasma renin activity as a stand-alone screening test is not reliable for diagnosing or excluding renal artery disease. Elevated plasma renin activity may be present in approximately 15% of patients with essential hypertension. In addition, patients with bilateral disease or disease to a solitary functioning kidney may have normal or low plasma renin activity due to extracellular volume expansion, position of the patient during the test, or medication use. The test is less accurate in azotemic patients and in African American patients.39 The captopril test (plasma renin measurement before and after administration of captopril) is not an ideal screening test and is rarely used. Renal vein renin measurement is not a useful test to screen for RAS; in addition, it has little value in determining who will benefit from revascularization. Except under unusual circumstances, this test is rarely used to make clinical decisions.
Captopril Scintigraphy
Radionuclide imaging techniques are a noninvasive and safe way of evaluating renal blood flow and excretory function, but the renal flow scan has unacceptably high false-positive and false-negative rates for diagnosing RAS.40 When an ACE inhibitor such as captopril is added to isotope renography, sensitivity and specificity of the test improve considerably, especially for patients with unilateral RAS. In most instances of unilateral RAS, the glomerular filtration rate (GFR) of the stenotic kidney falls by approximately 30% after captopril administration.41,42 In contrast, the contralateral normal kidney exhibits an increase in GFR, urine flow, and salt excretion despite a reduction in systemic blood pressure. These expected physiological changes within the stenotic and contralateral kidneys are the basis of the asymmetry of renal function following ACE inhibition detected by renal scintigraphy (see Chapter 22).43–45
In patients with normal renal function and unilateral disease, captopril renography has a sensitivity of around 85% to 90% (range 45-94) and specificity around 93% to 98% (range 81-100).46 However, the presence of significant azotemia or bilateral RAS may adversely affect the accuracy of captopril renography. Many investigators have excluded patients with a serum creatinine exceeding 2.5 to 3.0 mg/dL.47 Although the captopril renogram was once the noninvasive diagnostic test of choice for patients with RAS, it is now rarely used because the quality of the images of duplex ultrasound, magnetic resonance angiography (MRA), and computed tomographic angiography (CTA) are excellent, as discussed later.
Imaging Modalities to Detect Renal Artery Stenosis
Although catheter-based renal angiography with pressure gradient measurements is the definitive gold standard of RAS assessment, several noninvasive imaging modalities such as duplex ultrasound, CTA, and MRA, have become more practical first-line tests for the diagnosis of RAS. Imaging has become so sophisticated and accurate, it is seldom necessary to perform catheter-based angiography for the diagnosis of renal artery disease, and it usually is reserved for imaging at the time of percutaneous revascularization. The ideal imaging procedure should48:
1. Identify the main renal arteries as well as accessory or polar vessels.
2. Localize the site of stenosis or disease.
3. Determine the type of disease present (e.g., atherosclerosis, FMD).
4. Provide evidence for the hemodynamic significance of the lesion.
5. Determine the likelihood of a favorable response to revascularization.
6. Identify associated pathology (i.e., abdominal aortic aneurysm, renal mass, etc.) that may have an impact on the treatment of the renal artery disease.
7. Detect restenosis after percutaneous or surgical revascularization.
Duplex ultrasonography, CTA, and MRA do not by themselves fulfill all these criteria. Local expertise and availability, as well as economic costs, often dictate the preferred imaging modality used (Fig. 23-3). Factors that may play a role in determining the optimal screening test include the patient’s renal function, body habitus, and personal preference (e.g., claustrophobia).
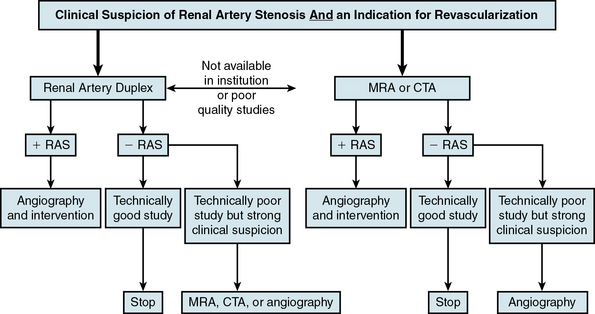
Figure 23-3 Algorithm for diagnosis of renal artery stenosis (RAS).
CTA, computed tomographic angiography; MRA, magnetic resonance angiography.
(Adapted from Carman T, Olin JW: Diagnosis of renal artery stenosis: what is the optimal diagnostic test? Curr Interv Cardiol Rep 2:111–118, 2000.)48
Duplex Ultrasonography
Duplex ultrasonography (also see Chapter 12), which is composed of real-time brightness (B-mode/gray scale) imaging and color pulsed-wave Doppler, has the advantages of being noninvasive, the least expensive of the imaging modalities, and provides both anatomical and functional information about the arterial segments being evaluated. Duplex ultrasonography also does not require the use of potentially nephrotoxic agents.
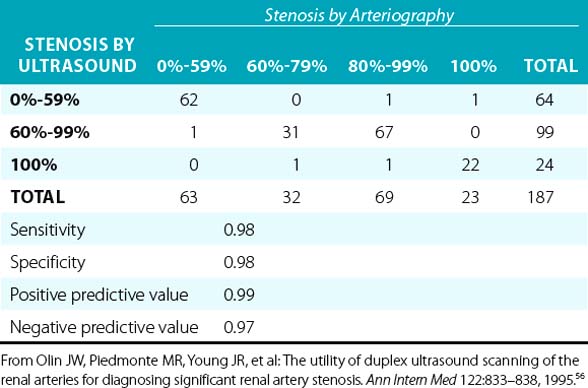
Overall, when compared to angiography, duplex ultrasonography has a sensitivity and specificity of 84% to 98% and 62% to 99%, respectively, when used to diagnose RAS.49–55 In a prospective blinded study, there was a very good correlation between duplex ultrasonography and angiography (Table 23-2). In addition, it was determined that if the end-diastolic velocity (EDV) was 150 cm/s or greater, the degree of stenosis was likely to be 80% or more.56
Renal artery ultrasound should be performed from both an anterior and oblique (or posterior [flank]) approach (Fig. 23-4). In the longitudinal view, the peak systolic flow velocity in the aorta is recorded at the level of the renal arteries. The renal-to-aortic ratio (RAR), which is the ratio of the highest peak systolic value (PSV) in the renal arteries to the PSV in the aorta, can then be calculated to help classify the degree of stenosis (see Table 23-3).54,56
Table 23-3 Duplex Criteria for Diagnosis of Renal Artery Stenosis
| RAR <3.5 and PSV <200 cm/s | 0%-59% |
| RAR ≥3.5 and PSV >200 cm/s | 60%-99% |
| RAR >3.5 and EDV ≥150 cm/s | 80%-99% |
| Absent flow, low-amplitude parenchymal signal | Occluded |
EDV, end-diastolic velocity; PSV, peak systolic velocity; RAR, renal-to-aortic ratio.
The renal arteries are best visualized in a transverse (short-axis) view. Using the B-mode image and a 60-degree angle of insonance, the arteries are interrogated with pulsed wave Doppler. The Doppler should be swept through the artery from its origin to the renal hilum, which will allow the examiner to survey the artery for velocity shifts along the entire course of the renal artery. Velocities should be recorded at the origin, proximal, mid-, and distal arterial segments. From an oblique approach, the renal artery can be visualized at the renal hilum and followed to the aorta. By studying the patient from an anterior and an oblique approach, Doppler velocity measurements are obtained in two views, assuring that a focal stenosis is not missed and that the angle of insonation is correct. Since medial fibroplasia most often occurs in the mid- to distal renal artery, the oblique approach is particularly good for detecting this type of stenosis. It is important to note that segmental Doppler interrogation (spot-checking) of the renal artery velocities is inadequate and often leads to an inaccurate result.54,56,57 When there is a discrepancy in kidney size of 1.5 cm or greater, the ultrasonographer should search very carefully for the presence of RAS or an occluded renal artery.
A three-category classification scheme based on the PSV within the proximal segment of the renal arteries is commonly used: 0% to 59% stenosis; 60% to 99% stenosis, and total occlusion. If the PSV is greater than 200 cm/s and turbulence is present in color Doppler flow, the stenosis would be classified as 60% to 99%. In the presence of a severe stenosis, there may be characteristic spectral broadening of the Doppler arterial waveform or parvus-tardus waveform just distal to the lesion. In addition to the PSV, the RAR is also used to help classify the degree of stenosis (Table 23-2). The caveat is that the RAR is not an accurate representation of the degree of stenosis when the aortic velocity is less than 40 cm/s or greater than 100 cm/s, or when an abdominal aortic aneurysm, or aortic stent graft is present.
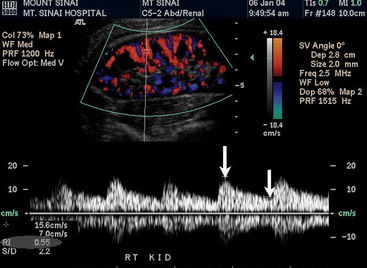
There are two other important advantages of duplex ultrasonography. First, duplex ultrasonography may help identify patients who will have a favorable clinical outcome after surgical or catheter-based renal revascularization.58 The RI is calculated as follows: [1-(end-diastolic velocity/peak systolic velocity)] × 100 (Fig. 23-5). Using a zero-degree angle of insonation, the peak systolic velocity and EDV are measured within the parenchyma of the kidney. Two studies help support use of the RI. A prospective study followed 138 patients with more than 50% RAS who underwent renal artery angioplasty or surgery for blood pressure control or preservation of renal function. A renal RI of 80 or greater identified patients in whom angioplasty or surgery was not associated with improved blood pressure, renal function, or kidney survival. Ninety-seven percent of patients with an increased renal RI demonstrated no improvement in blood pressure, and 80% had no improvement in renal function. The authors suggested that the increased RI identifies structural abnormalities in the small vessels of the kidney. Such small-vessel disease is typical of long-standing hypertension associated with nephrosclerosis or glomerulosclerosis.59 Similar conclusions were drawn from a more recent study that retrospectively evaluated the significance of associating preprocedural RI with postintervention outcomes (endovascular or open surgical repair for RAS treatment). Crutchley et al. found that a preprocedural RI of 0.8 or higher was highly associated with a postprocedural decline in renal function, and that the RI was also highly predictive of all-cause mortality.60 However, not all investigators believe RI is an accurate predictor of response to renal artery revascularization. A prospective study of renal stent placement in 241 patients demonstrated that individuals with an elevated RI (> 80) achieved a favorable blood pressure response and renal functional improvement a year after renal arterial intervention.61,62 Zeller et al. demonstrated that patients with the most abnormal RI values experienced the greatest magnitude of benefit.61 Until more information becomes available, an elevated RI should not be considered a contraindication to performing renal artery revascularization.63
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree