Chapter 38 Clinical Evaluation of Aortic Aneurysms
The vast majority of aortic aneurysms are asymptomatic, accounting for a much higher disease prevalence than hospitalization and mortality statistics would suggest (see Chapter 37). These data underscore the central challenge in aortic aneurysmal disease: a common clinical problem that is silent until rupture and death. Aortic aneurysms typically increase in size slowly over years or decades, with few warning signs. The management of aortic aneurysmal disease, therefore, requires suspicion and diligence to avoid adverse outcomes. This chapter will focus on the history, physical examination, and diagnostic tests important to clinical evaluation of aortic aneurysms.
Clinical History
Thoracic Aortic Aneurysms
Aneurysms of the aortic arch may produce symptoms by compression of contiguous structures, but most are asymptomatic. Dyspnea or cough may be caused by compression of the trachea or mainstem bronchi, dysphagia by compression of the esophagus, or hoarseness secondary to left vocal cord paralysis related to compression of the left recurrent laryngeal nerve. The superior vena cava syndrome and pulmonary artery stenosis result when these vessels are compressed.1,2 Chest pain, related either to compression of adjacent structures or to erosion of ribs or vertebrae, is typically positional. Aneurysms of the aortic arch may rupture into the mediastinum, pleural space, tracheobronchial tree (causing hemoptysis), or esophagus (causing hematemesis). Arteriovenous fistulas (AVFs) may result from rupture into the superior vena cava or pulmonary artery. Tuberculous aneurysms, akin to other causes of thoracic aortic aneurysms (TAAs), may present with pain but are commonly asymptomatic or may present with hypovolemic shock as a consequence of rupture.
Abdominal Aortic Aneurysms
In the absence of patient complaints, physicians must intuit the presence of aneurysm based on the clinical characteristics of the patient. Risk factors for aortic aneurysm disease (see Chapter 37) can be used to guide directed physical examination and, if necessary, diagnostic testing.
Physical Examination
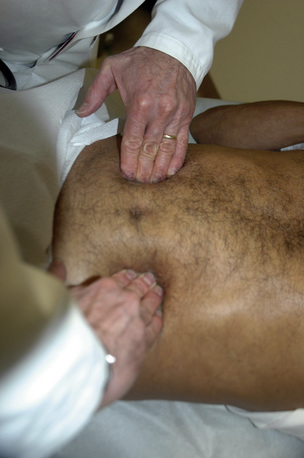
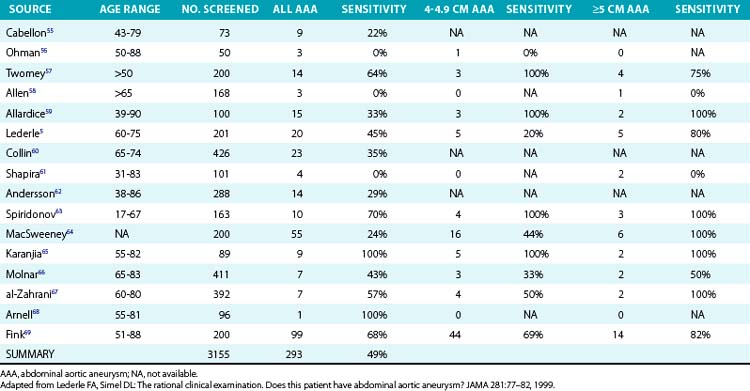
The key physical finding for an AAA is a pulsatile abdominal mass. The patient should be positioned supine with the knees flexed. A pulsatile epigastric or periumbilical mass may be visible as well as palpable. To distinguish an AAA from paraaortic masses requires that the examiner’s hands address the lateral borders (Fig. 38-1). An aneurysm expands laterally with each systole. This technique also permits estimation of the transverse diameter of the aneurysm. Auscultation may reveal a bruit over the mass, but abdominal bruits are not specific for aneurysm formation, and only about 40% of such aneurysms are associated with bruits. Proper physical examination of the abdomen may detect the presence of an AAA in 30% to 48% of patients with AAA.3,4 In a review of 15 studies, sensitivity of abdominal examination for aneurysm detection was 49%4 (Table 38-1).
Several factors limit the potential for AAA diagnosis. First, palpation for an aneurysm requires consideration of the diagnosis prior to examination. Routine physical examination decreases the sensitivity of the exam. Second, size matters; as the size of an aneurysm increases, so does the likelihood of making the diagnosis. Sensitivity of palpation increases to 75% in patients with aneurysms greater than 5 cm in diameter.4 Third, increasing abdominal girth decreases the likelihood of discovery. In one study of 201 patients, all six aneurysms present were diagnosed in patients with an abdominal girth less than 100 cm, but only 3 of 12 were detected when the abdominal girth exceeded 100 cm.5 So although the directed physical examination has moderate sensitivity and specificity for AAA diagnosis, routine examination misses the diagnosis more commonly than making it.
Screening and Surveillance of Aortic Aneurysms
Several trials have evaluated the possibility of reducing AAA event rates as a result of screening. In a study from Chichester, United Kingdom, 15,775 men and women aged 65 to 80 years were divided in two, and half were invited for an abdominal ultrasound screening.6 Nearly 70% of those offered screening accepted the invitation, and aneurysm was detected in 4%. A 55% reduction in rate of aneurysm rupture (2.8 per 1000 vs. 6.2 per 1000 subjects) and a 42% reduction in AAA-related mortality in men only (3 per 1000 vs. 5.3 per 1000 male subjects) were noted in the screening group compared to controls. A similar study in Denmark offered 12,658 65- to 73-year-old males a screening invitation, of whom 9620 accepted and 3038 declined.7 There was a tenfold reduction in AAA-related death in the group that accepted the invitation compared with those who did not (.006% vs. .06%). Only one trial has assessed the impact of screening on total mortality. The Multicentre Aneurysm Screening Study (MASS) assessed the impact of AAA screening in 67,800 men aged 65 to 74 years.8 Half were invited for AAA screening, and the others were not. Long-term mortality was monitored in both groups. In the screened group, there was a 42% relative risk reduction in aneurysm-related mortality from 0.33% to 0.19%, representing 48 fewer deaths. Reduction in absolute mortality, however, was a statistically insignificant 0.27%.
Some clinical features exist to suggest which patients should definitely undergo screening for aortic aneurysm, and AAA in particular. These include patients with a family history for aneurysm and inherited disorders of connective tissue (e.g., Marfan’s syndrome [MFS]) and those with arteritis (e.g., Takayasu and giant cell arteritis [GCA]). Siblings of patients with an AAA have a 25% chance of having an aneurysm.9
On the basis of these data, the American College of Cardiology/American Heart Association (ACC/AHA) Practice Guidelines for the Management of Peripheral Arterial Disease recommends that “Men 60 years of age or older who are either the siblings or offspring of patients with AAAs” and “men who are 65 to 75 years of age who have ever smoked should undergo a physical examination and one-time ultrasound screening for detection of AAAs.”10 The U.S. Preventive Services Task Force recommends one-time screening for AAA by ultrasonography in men aged 65 to 75 who have ever smoked, but does not recommend routine screening in women.11 Medicare will pay for a one-time ultrasound screen during the “Welcome to Medicare” preventive visit if the patient has a family history of AAA or is a man aged 65 to 75 who has smoked at least 100 cigarettes in his lifetime.
Screening for TAAs is less well established. Recent data have shown that bicuspid aortic valve (BAV) disease is an autosomal dominant condition that may be associated with TAA formation. Interestingly, patients with this condition may manifest the valvular or aneurysmal findings alone or in tandem.12 Thus, the ACC/AHA thoracic aortic disease guidelines recommend that all patients with a BAV should have both the aortic root and ascending thoracic aorta evaluated for evidence of aortic dilatation. In pedigree analysis of more than 500 patients with TAA, Albornoz et al. have shown that one in five non-MFS patients have an inherited pattern of disease.13
Because of the frequency of both known and unknown genetic conditions associated with TAA, the ACC/AHA thoracic aortic disease guidelines recommend that first-degree relatives of patients with a bicuspid aortic valve, premature onset of thoracic aortic disease with minimal risk factors, and/or a familial form of TAA and dissection should be evaluated for the presence of a BAV and asymptomatic thoracic aortic disease.14
Diagnostic Testing
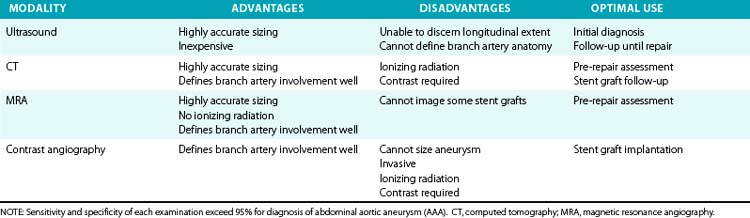
Major objectives of imaging studies include identifying the aorta and its branches, diagnosing and characterizing the type of aneurysm (fusiform or saccular), determining the transverse and longitudinal dimensions of the aneurysm, and detecting associated pathology that may affect treatment. Imaging studies are also indicated for longitudinal surveillance of known aneurysms, or for anatomical definition before endovascular or surgical repairs. An understanding of the benefits and limitations of the several imaging modalities will enable appropriate test selection (Table 38-2).
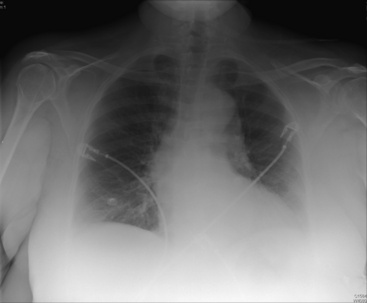
Chest roentgenography may provide the first indication of a TAA (Fig. 38-2). Aneurysms of the ascending thoracic aorta are usually evident on the right side of the mediastinum. Aneurysms of the aortic arch widen the mediastinal shadow and may project more toward the left. These aneurysms may displace or compress the trachea or left mainstem bronchus. Descending TAAs typically appear as mediastinal masses extending into the left hemithorax. Assessment of the aorta by chest roentgenography requires both posteroanterior and lateral projections. Failure to detect TAA roentgenographically, however, does not exclude the diagnosis, since aneurysms may not become apparent until considerable dilation has occurred.
Similarly, plain abdominal roentgenography frequently discloses an unsuspected AAA.3 Anteroposterior and lateral views of the abdomen may disclose a curvilinear rim of calcification in the wall of the aneurysm, and the diameter of the aneurysm may be estimated when such calcification is visible in two opposing walls. In 25% to 50% of suspected cases, however, the walls of the aneurysm are not sufficiently calcified to permit radiographic identification. Furthermore, it may underestimate anteroposterior aneurysm size by 15%.
Ultrasound
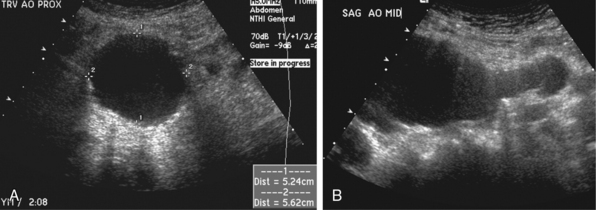
Ultrasonography is the most commonly used method for identification and characterization of AAA. It is the least expensive modality, does not expose the patient to ionizing radiation, and can accurately determine the anteroposterior, transverse, and longitudinal dimensions of an AAA (Fig. 38-3). Sensitivity for diagnosis of an AAA 3 cm or larger approaches 100%.10 The examination is rapid and easily performed. The abdominal aorta is subject to anteroposterior, transverse, and longitudinal evaluation. Sonographic classification of AAA begins when the maximum diameter exceeds 3 cm in either anteroposterior or transverse dimensions. Care must be taken to image the aorta perpendicular to its longitudinal axis to avoid eccentricity, which may lead to overestimating its true diameter. Thrombus is frequently identified within the lumen, and echodense calcification may be present in or adjacent to the aortic wall. Beyond determining the size of an aneurysm, ultrasound imaging may help define the relation of major arterial branches and adjacent organs. Certain ultrasound characteristics have potential value in predicting rupture. Intramural hematoma, appearing as a hypoechoic soft-tissue mass surrounding the aorta that may silhouette the psoas muscle, appears to represent such a sign.15 This may be indistinguishable from periaortic fibrosis, which appears on ultrasound examination as a hypoechoic mantle surrounding the aortic wall in patients with inflammatory aortic aneurysms.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree