Chapter 27 Clinical Evaluation and Treatment of Mesenteric Vascular Disease
Evaluation
Acute Occlusive Mesenteric Ischemia
Signs and symptoms
Acute occlusive mesenteric ischemia is caused by embolism to the superior mesenteric artery (SMA) or acute thrombotic occlusion of an atherosclerotic SMA. Mortality exceeds 60%.1
Radiological diagnosis
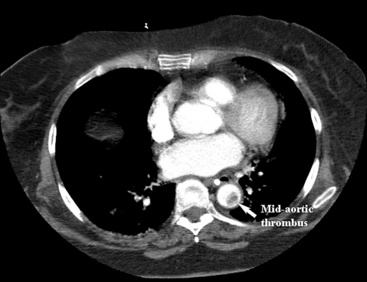
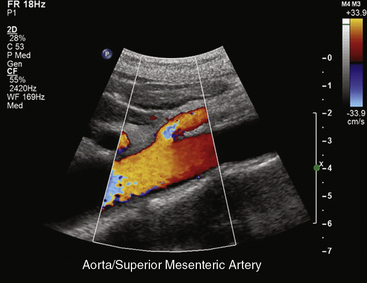
An abdominal computed tomography (CT) scan is often obtained during evaluation of a patient with abdominal pain. In addition to finding other abdominal pathologies causing abdominal pain, CT scans can detect late findings of acute mesenteric ischemia with a sensitivity of 90%, including bowel luminal dilation, bowel wall thickening, submucosal edema or hemorrhage, pneumatosis intestinalis, and portal venous gas.2 These findings are associated with some degree of intestinal infarction. Emboli tend to lodge distally, and thus the sensitivity of CT scanning in detecting mesenteric arterial embolic occlusion is low and ranges from 37% to 80%3 (Fig. 27-1). In patients with early ischemia, there may be minimal findings on CT scans. These patients benefit most from early diagnosis and definitive mesenteric revascularization before the onset of intestinal infarction. Therefore, a high index of suspicion and thorough physical examination is of the utmost importance in this population of patients.
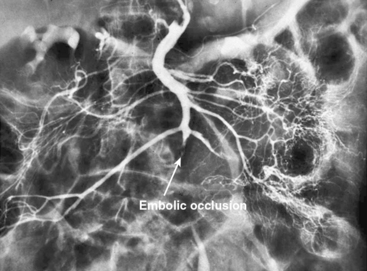
In patients without peritoneal findings but clinically suspected to have mesenteric ischemia, angiography can be performed to make the definitive diagnosis and plan additional therapy. Abrupt occlusion of the SMA distal to the origin of the middle colic artery is typically seen in patients with an embolus (Figs. 27-2 through 27-4), whereas patients with thrombotic occlusion of a chronically stenotic SMA show signs of occlusion beginning at its origin from the aorta.
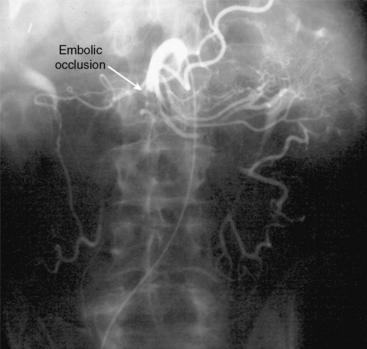
Figure 27-2 Anteroposterior angiogram demonstrating abrupt embolic occlusion of superior mesenteric artery (SMA).
Treatment
Operative Embolectomy
Once angiography has identified embolic disease, the patient is taken to the operating room for abdominal exploration and embolectomy. When performing an SMA embolectomy, a midline incision in the abdomen is made, followed by a thorough examination of the abdominal contents. This may or may not reveal intestinal infarction. The transverse colon is then reflected superiorly, and the SMA is approached at the root of the small-bowel mesentery. The ligament of Treitz is divided and the proximal SMA mobilized. If the embolus is located more distally, the distal SMA may be exposed at the root of the small-bowel mesentery. Embolectomy is performed through a transverse arteriotomy using standard balloon catheters, and the embolus is extracted. The arteriotomy is then closed, and the intestines are again inspected for viability; any nonviable bowel is resected. A Doppler probe can be used to assess the antimesenteric border for intestinal arterial flow. If the bowel viability is equivocal, a “second look” operation can be planned in the following 24 to 48 hours to reassess the bowel and resect if necessary.4
Chronic Mesenteric Ischemia
Radiological diagnosis
Mesenteric Angiography
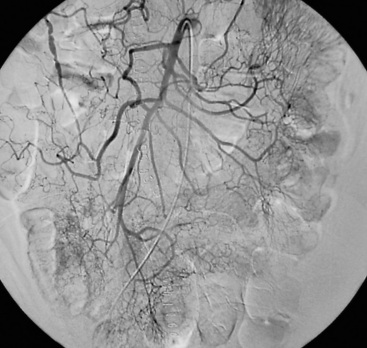
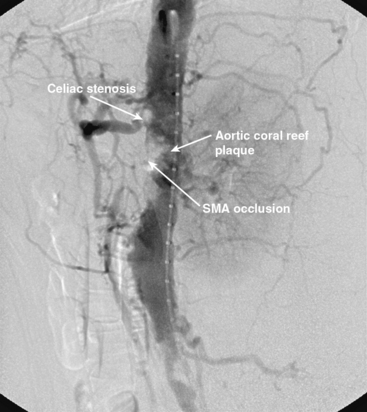
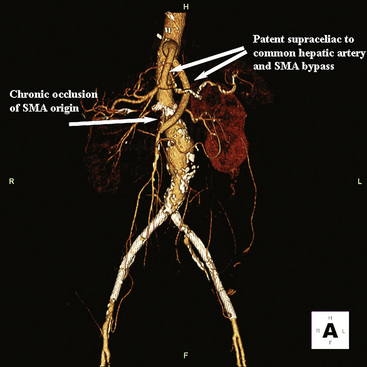
Chronic mesenteric ischemia is a clinical diagnosis. There are no absolute confirmatory tests, but contrast mesenteric angiography is the standard to diagnose arterial lesions associated with CMI. Radiographs are obtained in the lateral and anteroposterior projections. Findings on angiography suggestive of CMI include stenosis or occlusion of the celiac artery (CA) and/or the SMA (Fig. 27-5). When the origins of the CA or SMA are occluded, the more distal vessels are often patent through filling by enlarged and easily visualized pancreaticoduodenal arterial collaterals. Occasionally, one can find mid-SMA stenosis with a normal proximal SMA (Fig. 27-6) or a “coral reef” aorta with associated SMA occlusion (Fig. 27-7). Not all patients with mesenteric artery obstruction, however, have mesenteric ischemia. It is vitally important to differentiate high-grade mesenteric artery stenosis from the clinical entity of CMI.
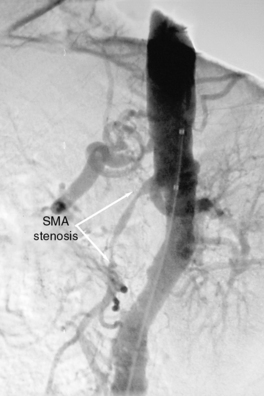
Figure 27-5 Lateral aortogram demonstrating long-segment stenosis of superior mesenteric artery (SMA).
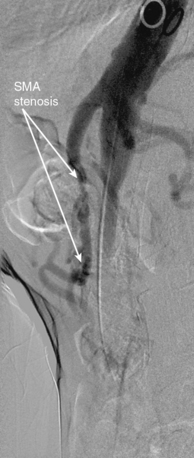
Figure 27-6 Lateral aortogram demonstrating long-segment stenosis of middle superior mesenteric artery (SMA).
Duplex Ultrasonography
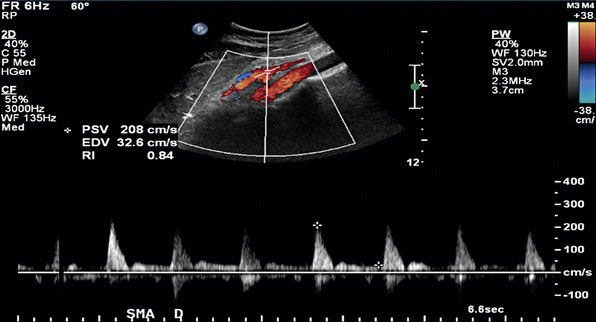
In healthy individuals, fasting blood flow velocity waveforms differ between the SMA and the CA. Arterial waveforms reflect end-organ vascular resistance. The liver and spleen have relatively high constant metabolic requirements and are therefore low-resistance organs. As a result, CA waveforms are generally biphasic with a peak systolic component, no reversal of end-systolic flow, and a relatively high end-diastolic velocity (EDV). The normal fasting SMA velocity waveform is triphasic, reflecting the high vascular resistance of the intestinal tract at rest (Figs. 27-8 and 27-9). There is a peak systolic component, often an end-systolic reverse flow component, and a minimal diastolic flow component.
Changes in Doppler-derived arterial waveforms in response to feeding are different in the CA and SMA. Because the liver and spleen have fixed metabolic demands, there is no significant change in CA velocity waveform after eating. Blood flow in the SMA, however, increases markedly after a meal, reflecting a marked decrease in intestinal arterial resistance. Waveform changes in the SMA postprandially include a near doubling of systolic velocity, tripling of the EDV, and loss of end-systolic reversal of blood flow. In addition, there is a small but detectable increase in the diameter of the SMA postprandially. The diameter of the SMA has been shown to be 0.60 ± 0.09 cm in the fasting state and 0.67 ± 0.09 cm after a meal. These changes are maximal at 45 minutes after ingestion of a test meal5 and are dependent on the composition of the meal ingested. Mixed composition meals produce the greatest flow increase in the SMA when compared with equal caloric meals composed solely of fat, glucose, or protein.6
Detection of Splanchnic Arterial Stenosis
Duplex ultrasound can detect hemodynamically significant stenoses in splanchnic vessels. In 1986, investigators at the University of Washington found that flow velocities in stenotic SMAs and CAs were significantly increased when compared with normal SMAs and CAs.7 Quantitative criteria for splanchnic artery stenosis were first developed and validated at Oregon Health & Science University.8
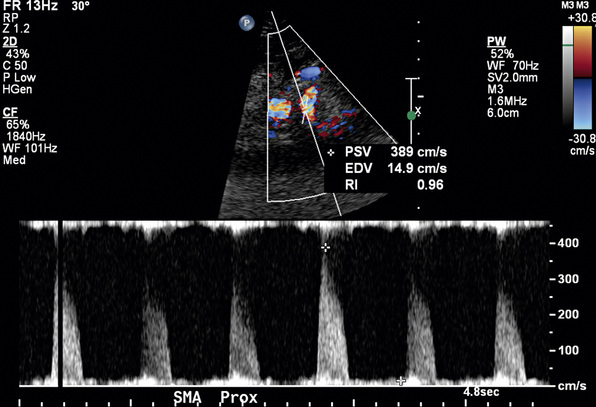
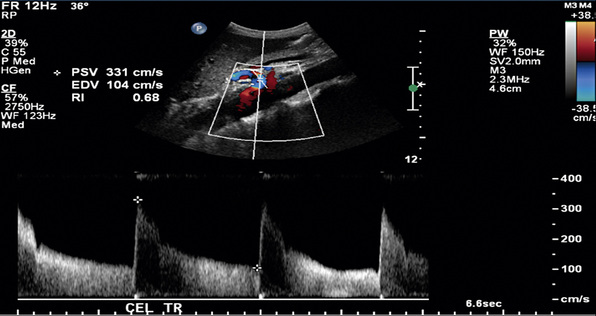
In a blinded prospective study of 100 patients who underwent mesenteric artery duplex scanning and lateral aortography, a peak systolic velocity (PSV) in the SMA of 275 cm/s or more indicated 70% or greater SMA stenosis with a sensitivity of 92%, specificity of 96%, positive predictive value of 80%, negative predictive value of 99%, and accuracy of 96%9 (Fig. 27-10). In the same study, a PSV of 200 cm/s or higher identified 70% or greater angiographic CA stenosis with a sensitivity of 87%, specificity of 80%, positive predictive value of 63%, negative predictive value of 94%, and accuracy of 82% (Fig. 27-11).
Other duplex criteria for mesenteric artery stenoses are also in use. An SMA EDV greater than 45 cm/s correlates with 50% or greater SMA stenosis with 92% specificity and 100% sensitivity. A CA EDV of 55 cm/s or higher predicts 50% or greater CA stenosis with 93% sensitivity, 100% specificity, and 95% accuracy.10,11
Postprandial mesenteric duplex scanning has been used as an adjunct to fasting duplex scanning to aide in the diagnosis of mesenteric artery stenoses.12 In patients with less than 70% SMA stenosis, postprandial SMA PSV increases by more than 20% over baseline velocity. The percent increase in SMA PSV is less in patients with 70% or greater SMA stenosis. Specificity for the combination of fasting SMA PSV and postprandial PSV, however, is marginally improved over that provided by a fasting duplex scan alone. Therefore, although theoretically attractive, postprandial duplex scanning offers no significant improvement over fasting mesenteric duplex scanning; its routine use as part of ultrasound assessment of mesenteric artery stenosis is unnecessary. Postprandial examinations are occasionally useful in technically difficult ultrasound studies in that if there is a postprandial response, the insonated vessel can be confirmed as being the SMA.
Computed Tomography
Standard CT imaging can be of some value in mesenteric artery stenosis evaluation (also see Chapter 14). Currently, however, no studies have compared the accuracy of spiral CT with angiography in assessing visceral artery stenoses. Computed tomography angiography can detect but not precisely quantify proximal stenosis of the CA and SMA, and it is limited by lower resolution and bowel motion artifact in its ability to detect lesions involving more distal branches.13 Computed tomography scans are often obtained to evaluate abdominal pain. Findings suggestive of mesenteric artery stenosis include calcification at the origin of the CA and SMA and lack of contrast enhancement within the vessel lumen (Fig. 27-12).
Magnetic Resonance Imaging
Development of fast breath-hold three-dimensional gadolinium-enhanced magnetic resonance angiography (3D Gd-enhanced MRA) has improved the ability of MRA to detect proximal splanchnic artery lesions (also see Chapter 13). Magnetic resonance angiography is limited in its ability to image more distal visceral branches because of limited spatial resolution, peristaltic and respiratory motion, and chemical shift changes between the vessels and fat. The accuracy of 3D Gd-enhanced MRA in detecting visceral artery stenosis has been well studied. Overall sensitivity and specificity for detecting 75% or greater stenosis or occlusion of the CA, SMA, or inferior mesenteric artery (IMA) were 100% and 95%, respectively.14
In addition to providing anatomical details, magnetic resonance (MR) technology can quantify arterial flow using the phase-contrast magnetic resonance imaging (MRI) technique, which provides information about the presence, magnitude, and direction of blood flow and has been studied in patients with CMI.15 Fasting and postprandial flow in the SMA have been compared in patients with documented atherosclerotic disease and normal volunteers. Mean fasting SMA blood flow has been shown to be higher in atherosclerotic patients than in normal individuals. This difference can be used to evaluate for CMI. The addition of MR oximetry technology, where increased oxygen extraction after a meal is seen in those with significant mesenteric vessel atherosclerosis, is promising with regard to diagnosing CMI.15 Further validation studies correlating the degree of change in mesenteric blood flow and oxygenation with angiographic percentage of stenosis will be necessary before phase-contrast MRI and MR oximetry are universally adopted as routine noninvasive diagnostic methods for patients with CMI.
Treatment
Chronic and Acute Mesenteric Occlusive Disease
The available literature includes no randomized or controlled clinical trials of surgical intervention for treatment of mesenteric ischemia, and none are likely to be performed. Published clinical reports include varied recommendations for treatment, and many do not include descriptions of operative methods. Others describe technically demanding procedures requiring extensive dissections in difficult areas.16,17 Only recently has objective determination of postoperative graft patency been included in clinical series.18,19 For all these reasons, there is no current consensus regarding the surgical details of treatment for intestinal ischemia.
History
In 1936, Dunphy reviewed the medical records of 12 patients dying from intestinal ischemia and discovered that more than half (58%) had evidence of chronic abdominal pain.20 This finding suggested that timely surgical intervention may have prevented progression to intestinal infarction and death. In 1957, Mikkelsen described the arteriographic appearance of typical orificial atherosclerotic lesions affecting the mesenteric arteries. That same year, the first successful surgical procedure (SMA endarterectomy) for treatment of chronic intestinal ischemia was performed by Maynard and Shaw.21
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree