We examined the relations between right bundle branch block (RBBB) and clinical characteristics, management, and outcomes among a broad spectrum of patients with acute coronary syndrome (ACS). Admission electrocardiograms of patients enrolled in the Global Registry of Acute Coronary Events (GRACE) electrocardiogram substudy and the Canadian ACS Registry I were analyzed independently at a blinded core laboratory. We performed multivariable logistic regression analysis to assess the independent prognostic significance of admission RBBB on in-hospital and 6-month mortality. Of 11,830 eligible patients with ACS (mean age 65; 66% non–ST-elevation ACS), 5% had RBBB. RBBB on admission was associated with older age, male sex, more cardiovascular risk factors, worse Killip class, and higher GRACE risk score (all p <0.01). Patients with RBBB less frequently received in-hospital cardiac catheterization, coronary revascularization, or reperfusion therapy (all p <0.05). The RBBB group had higher unadjusted in-hospital (8.8% vs 3.8%, p <0.001) and 6-month mortality rates (15.1% vs 7.6%, p <0.001). After adjusting for established prognostic factors in the GRACE risk score, RBBB was a significant independent predictor of in-hospital death (odds ratio 1.45, 95% CI 1.02 to 2.07, p = 0.039), but not cumulative 6-month mortality (odds ratio 1.29, 95% CI 0.95 to 1.74, p = 0.098). There was no significant interaction between RBBB and the type of ACS for either in-hospital or 6-month mortality (both p >0.50). In conclusion, across a spectrum of ACS, RBBB was associated with preexisting cardiovascular disease, high-risk clinical features, fewer cardiac interventions, and worse unadjusted outcomes. After adjusting for components of the GRACE risk score, RBBB was a significant independent predictor of early mortality.
Right bundle branch block (RBBB) in the context of acute coronary syndrome (ACS) is not an infrequent occurrence, ranging from 1.6% to 15% in hospitalized patients. Several studies demonstrate increased mortality in this high-risk group despite advances in therapeutics and early revascularization strategies. It is widely known that RBBB after anterior myocardial infarction (MI), caused by complete occlusion of the proximal left anterior descending (LAD) artery, is a predictor of mortality. In patients presenting with RBBB, those with ST-segment elevation MI (STEMI) undergoing fibrinolysis or angioplasty have poorer short- and long-term prognosis than those without STEMI. Investigators have recently called for updated reperfusion guidelines to reflect the adverse prognosis of new RBBB in ACS, even in the absence of ST elevation. Patients with non–ST-elevation (NSTE) ACS comprise a heterogeneous group with variable prognoses that warrant early risk stratification to minimize adverse outcomes. NSTE-ACS with RBBB may predict worse outcomes because of more extensive underlying coronary artery disease as opposed to STEMI, where RBBB may reflect larger infarcts. Furthermore, previous studies were limited by small sample sizes usually from single centers, lacked blinded electrocardiogram (ECG) interpretation, and did not adjust for other independent prognosticators in validated risk scores. Therefore, the objective of our study was to determine the relation between presenting RBBB and clinical characteristics, in-hospital management, and clinical outcomes across a broad spectrum of patients with ACS, including NSTE-ACS and STEMI.
Methods
The Canadian ACS Registry I and Global Registry of Acute Coronary Events (GRACE) were prospective, multicenter, observational studies of the clinical characteristics, management, and outcomes of patients with NSTE-ACS and STEMI. Their rationale and design have been described elsewhere.
In brief, the ACS Registry I enrolled patients from September 1999 to June 2001 across 51 Canadian hospitals (n = 4,627). Eligible patients were aged ≥18 years and admitted to hospital for suspected ACS within 24 hours of symptom onset. ECGs from all patients were obtained at admission. GRACE included patients from 94 international sites aged ≥18 years and admitted to hospital with a presumed diagnosis of ACS based on ischemic cardiac symptoms and at least one of the following: ECG changes, elevated biomarkers, and/or documented history of coronary artery disease. For the present study, we included patients from the GRACE ECG substudy involving 39 sites in 11 countries from March 1999 to January 2004 (n = 7,900). Both registries excluded patients if their presenting condition was triggered by another major co-morbidity such as surgery, trauma, or gastrointestinal bleeding. All centers were encouraged to enroll consecutive patients to minimize selection bias.
All data on patient demographics, clinical presentation, investigations, management, and outcomes were recorded on standardized case report forms by local study coordinators or the responsible physician during index hospitalization. Forms for the ACS Registry were scanned into a central database (Teleform, version 7.0; Cardiff, San Diego, California) at the Canadian Heart Research Center in Toronto, Canada. GRACE data were managed by a coordinating center at the University of Massachusetts (Worcester, Massachusetts). Central data checks were executed and queries forwarded to participating centers for clarification of sampling protocols. After hospital discharge, patients were followed up through telephone interviews at 6 months in GRACE and 12 months in the ACS Registry to ascertain vital status. Study protocols were approved by local review boards, and all patients provided informed consent. Primary outcomes were in-hospital and cumulative 6-month all-cause mortality. Secondary outcomes included in-hospital myocardial (re)infarction (defined as new or recurrent beyond 24 hours of hospitalization), heart failure (only recorded in GRACE), and the composite of death or myocardial (re)infarction.
Admission ECGs were recorded at standard paper speed of 25 mm/s and calibration of 10 mm/mV and were forwarded to the Canadian Heart Research Center ECG core laboratory for systematic interpretation. ECGs were read by trained physicians blinded to clinical data, site interpretation, and patient outcomes. The core laboratory has previously demonstrated interobserver and intraobserver agreements of 93% to 99% and 100%, respectively. We defined ST-segment elevation as the presence of ≥0.1 mV ST-segment elevation in 2 contiguous leads; ST-segment depression as the presence of ≥0.05 mV ST-segment depression in ≥1 lead, excluding aVR; and T wave inversion as the presence of ≥0.1 mV deviation from the isoelectric baseline in 2 contiguous leads. Pathologic Q waves were defined as Q ≥30 ms in leads I, aVL, II, aVF; any Q in V1 to V3; Q ≥20 ms in V4; Q ≥30 ms in V5 to V6 when present in ≥2 contiguous leads. RBBB was coded if all the following criteria were met: (1) QRS duration of >120 ms in the presence of normal sinus or supraventricular rhythm; (2) R or RSR′ complex in lead V 1 ; and (3) RS in leads V 5 , V 6 , I, or aVL, with a prolonged shallow S wave. Patients with left bundle branch block, poor quality or incomplete ECGs, and ventricular-paced rhythms were excluded (n = 697).
Cardiac catheterization during the index hospitalization was undertaken at the discretion of the treating physician. Significant coronary artery stenosis was defined as ≥50% narrowing compared with the diameter of the adjacent normal segment, and 3-vessel disease as significant stenosis in all 3 epicardial coronary arteries (i.e., LAD, left circumflex, and right coronary artery) or their main branches. Angiographic data were only available for patients who underwent cardiac catheterization from the GRACE ECG substudy (n = 4,277). Those with previous coronary artery bypass grafting were excluded from the analysis of angiographic data.
Continuous variables are expressed as medians with interquartile ranges, and categorical variables as percentages. We used nonparametric Mann–Whitney U and Pearson chi-square tests to examine differences in continuous and categorical variables, respectively. We performed multivariable logistic regression analysis to evaluate the independent prognostic significance of RBBB during index hospitalization and at 6 months, adjusting for known predictors of the in-hospital GRACE risk model (i.e., age, heart rate, systolic blood pressure, cardiac arrest, Killip’s class, serum creatinine, initial cardiac biomarker elevation, and ST-segment deviation). The GRACE risk score for predicting cumulative 6-month mortality rate also included previous MI and heart failure. The GRACE risk score has previously demonstrated excellent discrimination in external validation cohorts. Model discrimination and calibration were evaluated by the c-statistic and Hosmer–Lemeshow goodness-of-fit test, respectively. We tested for an interaction effect between RBBB and the type of ACS (NSTE-ACS and STEMI) on mortality. All analyses were performed using SPSS, version 22, (IBM, Armonk, New York) and statistical significance was set at a 2-sided p value <0.05.
Results
Our study included 11,830 eligible patients with a mean (±SD) age of 65 (±13) years of whom 33% were women and 66% had NSTE-ACS. Overall, 590 patients (5%) had RBBB on the presenting ECG.
Baseline characteristics of the patients are listed in Table 1 . Patients with RBBB were older, more likely men, and had more cardiovascular co-morbidities. They also had higher rates of previous transient ischemic attack or stroke, congestive heart failure, peripheral vascular disease, and coronary artery disease. Patients with RBBB presented with higher heart rates and creatinine levels, worse Killip class; they more frequently had elevated initial cardiac biomarkers and had higher GRACE risk scores ( Table 2 ). They also were more likely to have ST depression, T-wave inversion, and Q waves in the precordial leads. However, patients with RBBB were less likely to present with ST elevation than those without RBBB.
| Variables | Right Bundle Branch Block | ||
|---|---|---|---|
| No (n = 11,240) | Yes (n = 590) | P value | |
| Age, (years) ∗ | 65 (55-74) | 73 (65-80) | < 0.001 |
| Men | 66.8% | 76.2% | < 0.001 |
| Systemic hypertension | 54.2% | 61.9% | < 0.001 |
| Dyslipidemia | 45.3% | 45.3% | 0.99 |
| Diabetes mellitus | 23.3% | 31.3% | < 0.001 |
| Current smoker | 30.0% | 22.4% | < 0.001 |
| Prior angina pectoris | 55.9% | 63.5% | < 0.001 |
| Prior myocardial infarction | 30.7% | 37.3% | 0.001 |
| Prior heart failure | 9.2% | 15.5% | < 0.001 |
| Prior percutaneous coronary intervention | 14.9% | 16.7% | 0.24 |
| Prior coronary bypass graft surgery | 11.1% | 19.9% | < 0.001 |
| Prior transient ischemic attack/stroke | 7.5% | 12.1% | < 0.001 |
| Prior peripheral vascular disease † | 9.0% | 14.3% | 0.001 |
∗ Median (25th to 75th percentiles).
| Variables | Right Bundle Branch Block | ||
|---|---|---|---|
| No (n=11,240) | Yes (n=590) | P value | |
| Systolic blood pressure, (mm Hg) ∗ | 142 (124-161) | 140 (124-164) | 0.81 |
| Diastolic blood pressure, (mm Hg) ∗ | 80 (70-91) | 80 (68-90) | 0.001 |
| Heart rate, (beats/min) ∗ | 75 (63-88) | 80 (65-94) | <0.001 |
| Killip Class I | 82.7% | 76.0% | <0.001 |
| Killip Class II | 13.8% | 18.1% | |
| Killip Class III | 2.9% | 4.9% | |
| Killip Class IV | 0.6% | 1.0% | |
| Creatinine, (μmol/L) ∗ | 90 (79-106) | 99 (83-127) | <0.001 |
| Elevated cardiac biomarkers | 41.8% | 45.3% | 0.10 |
| Any T-wave inversion(≥2 contiguous leads) | 27.7% | 36.3% | <0.001 |
| T-wave inversion in V1 and V2 | 8.6% | 32.7% | <0.001 |
| T-wave inversion in V2 and V3 | 8.4% | 23.9% | <0.001 |
| T-wave inversion in 2 adjacent precordial leads | 19.3% | 29.7% | <0.001 |
| Q wave in V1 and V2 | 8.2% | 11.5% | 0.005 |
| Q wave in ≥2 precordial leads | 14.4% | 23.4% | <0.001 |
| ST deviation (≥0.5 mm) | 78.7% | 79.0% | 0.86 |
| Any ST depression (>0.5 mm) | 53.2% | 61.0% | <0.001 |
| ST depression ≥0.5 mm in V1 and V2 | 5.5% | 10.0% | <0.001 |
| ST elevation ≥1 mm in ≥2 contiguous leads | 34.6% | 29.0% | 0.005 |
| ST elevation ≥1 mm in V1 and V2 | 4.8% | 3.2% | 0.081 |
| Cardiac arrest | 1.5% | 3.8% | <0.001 |
| GRACE risk score ∗ | 128 (104-153) | 143 (118-173) | <0.001 |
Table 3 summarizes in-hospital management for patients with and without RBBB. Patients with RBBB were less likely to receive fibrinolysis, cardiac catheterization, percutaneous coronary intervention, and coronary artery bypass grafting. Of 4,277 patients who underwent coronary angiography ( Figure 1 ), those with RBBB had higher rates of ≥50% stenosis in any coronary artery, left main stenosis, and triple vessel disease. Figure 2 summarizes the unadjusted clinical outcomes. The RBBB group had a lower left ventricular (LV) ejection fraction with a higher proportion of patients having moderately or severely diminished LV function compared to those without RBBB ( Table 3 ), and correspondingly higher rates of in-hospital heart failure. In-hospital (re)infarction rates were not significantly different between those presenting with and without RBBB.
| Variables | Right Bundle Branch Block | ||
|---|---|---|---|
| No RBBB (n = 11,240) | RBBB (n = 590) | P value | |
| Fibrinolysis | 19.5% | 16.2% | 0.047 |
| Cardiac catheterization | 51.3% | 44.2% | 0.001 |
| Percutaneous coronary intervention | 26.9% | 22.1% | 0.011 |
| Coronary bypass graft surgery | 3.9% | 4.3% | 0.63 |
| Percutaneous coronary intervention and/or coronary bypass graft surgery | 30.6% | 26.4% | 0.031 |
| Left ventricular function ∗ | < 0.001 | ||
| Normal | 56.2% | 43.1% | |
| Mildly impaired | 24.3% | 22.1% | |
| Moderately impaired | 17.3% | 29.5% | |
| Severely impaired | 2.2% | 5.4% | |

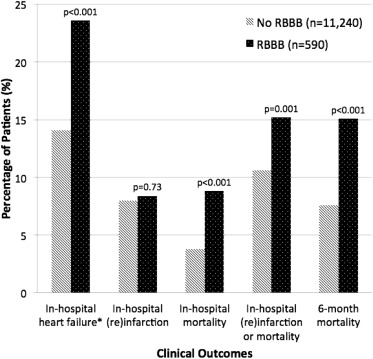
Overall, 485 patients (4.1%) died in hospital. Data on 6-month vital status were available for 89.1% of the study cohort; the 6-month mortality rate was 8.0%. Unadjusted rates of in-hospital mortality (8.8% vs 3.8%, p <0.001) and cumulative 6-month mortality rates (15.1% vs 7.6%, p <0.001) were almost doubled in the RBBB group compared to the non-RBBB cohort.
After adjusting for known prognostic factors in the GRACE risk models ( Table 4 ), RBBB was independently associated with in-hospital mortality (odds ratio [OR] 1.45 95% CI 1.02 to 2.07, p = 0.039). The adverse prognostic value of RBBB was consistently observed across the groups with NSTE-ACS and STEMI (p for interaction = 0.62). RBBB was numerically, but not statistically significantly associated with cumulative 6-month mortality rate (OR 1.29, 95% CI 0.95 to 1.74, p = 0.098; Table 5 ). The c-statistics were 0.85 and 0.83, and Hosmer–Lemeshow p values were 0.48 and 0.67, respectively, indicating good discrimination and calibration of the models. RBBB was not an independent predictor of the composite end point of in-hospital myocardial (re)infarction or death (OR 1.20, 95% CI 0.93 to 1.56, p = 0.17).



