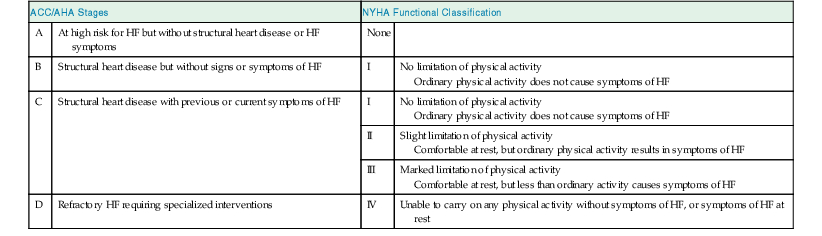
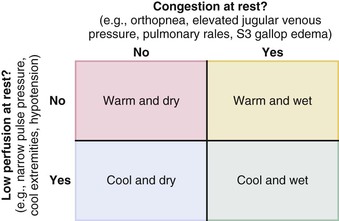
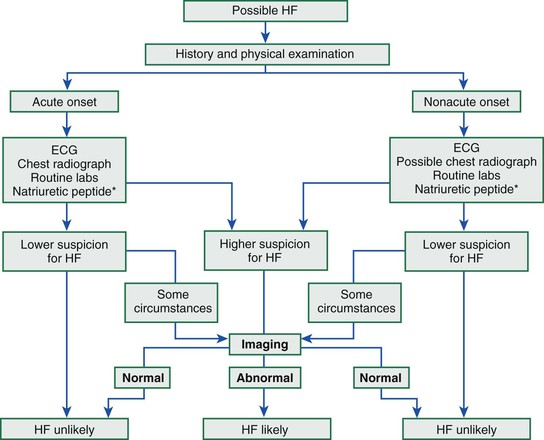
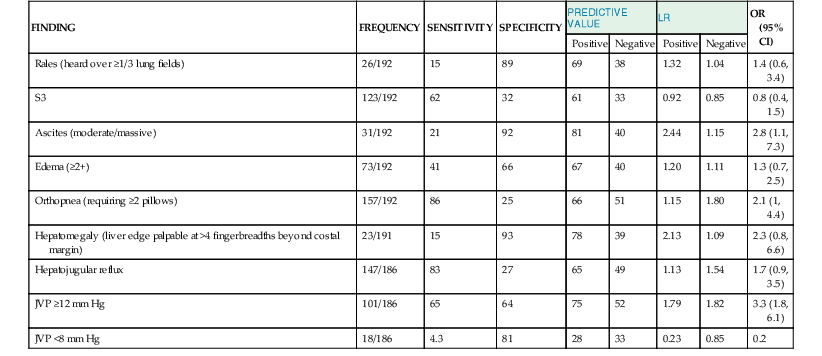
James L. Januzzi Jr.,, Douglas L. Mann Heart failure (HF) is a complex clinical syndrome resulting from structural and functional impairment of ventricular filling or ejection of blood. Although the clinical syndrome of HF may arise as a consequence of abnormalities or disorders involving all aspects of cardiac structure and function, most patients have impairment of myocardial performance, with findings ranging from normal ventricular size and function to marked dilation and reduced function. Although symptoms of HF frequently depend on the presence of elevated left- or right-sided heart filling pressures, the designation “congestive” in this context is no longer preferred, because many patients do not have overt congestion at the time of evaluation. Approximately half of the patients with HF have normal left ventricular function, that is, HF with preserved ejection fraction (HFpEF) (see Chapter 27); the balance have HF with reduced ejection fraction (HFrEF) (see Chapter 25]).1 HFpEF generally is defined as a left ventricular ejection fraction of 50% or greater, whereas HFrEF is defined as an ejection fraction below 40%. Because treatment strategies for treating HF are based on these two categories, these distinctions are crucial. Two useful methods to classify patients with HF are recognized. The American College of Cardiology/American Heart Association (ACC/AHA) HF staging approach (see Fig. 25-6) emphasizes the importance of development and progression of disease,2 (see Chapter 25G) whereas the New York Heart Association (NYHA) functional classification focuses more on exercise tolerance in persons with established HF (Table 23-1). Although suffering from considerable subjectivity, the NYHA functional classification is widely used. Use of both systems in conjunction provides a reasonable framework for clinician communication and patient prognostication. When HF is suspected, the goals of the clinical assessment are to determine whether HF is present, define the underlying cause, assess severity of the disease and the patient’s prognosis, and identify comorbid conditions that can influence the clinical course and response to treatment. When the diagnosis of HF has already been established, the goals are similar, with a particular focus on optimal therapeutic intervention. Although the diagnosis of HF can be straightforward when the patient presents with a constellation of the classic signs and symptoms in the appropriate clinical setting (Tables 23-2 and 23-3), no sign or symptom alone can define the presence or severity of HF. Furthermore, the detection of diagnostic physical findings in HF is an imprecise science, often requiring other diagnostic tools. Thus, as depicted in Figure 23-1, the clinical assessment of HF most often depends on information that is gleaned from a variety of sources including the history (both past and present), physical examination, laboratory tests, cardiac imaging, and functional studies. TABLE 23-2 Using the Medical History to Assess the Patient with Heart Failure (HF) Shortness of breath at rest or during exercise Edema (of the extremities or scrotum or elsewhere) Increasing abdominal girth or bloating Abdominal pain (particularly if confined to the right upper quadrant) Loss of appetite or early satiety Cheyne-Stokes respirations (often reported by a family member rather than the patient) TABLE 23-3 Physical Findings in Heart Failure * Indicative of more severe disease. A complete medical history and carefully focused physical examination are the foundation of the assessment of patients with HF, providing important information regarding etiology of HF, identifying possible exacerbating factors, and lending pivotal data for proper management (Chapter 11). The information obtained guides the further direction of the patient’s evaluation and enables the clinician to make the most judicious use of additional tests. Furthermore, the history helps to evaluate incongruent results that may emerge during the diagnostic process, and it can avoid needless further testing. Patients with HF may complain of a vast array of symptoms, the most common of which are listed in Table 23-1. Although none of these are entirely sensitive or specific for identifying the presence of severe congestion (Table 23-4), some are more reliable than others for this indication. None are specific to HFpEF versus HFrEF. TABLE 23-4 Sensitivity and Specificity of History and Physical Findings for Diagnosis of Elevated Filling Pressures in Patients with Heart Failure (HF)* * Values expressed as percent unless otherwise indicated. JVP = jugular venous pressure; LR = likelihood ratio; OR = odds ratio. Based on data from Drazner MH, Hellkamp AS, Leier CV, et al: Value of clinician assessment of hemodynamics in advanced heart failure: the ESCAPE trial. Circ Heart Fail 1:170, 2008. Worsening dyspnea is a cardinal symptom of HF and typically is related to increases in cardiac filling pressures but also may represent restricted cardiac output.3 The absence of worsening dyspnea, however, does not necessarily exclude the diagnosis of HF, because patients may accommodate symptoms by substantially modifying their lifestyle. Probing more deeply into the current level of activity may uncover a decline in exercise capacity that is not immediately apparent. Dyspnea at rest often is mentioned by patients hospitalized with HF and has a high diagnostic sensitivity and significant prognostic ramifications in this population. However, it also is cited by patients with many other medical conditions, so that the specificity and positive predictive value for dyspnea at rest alone are low. Patients may sleep with the head elevated to relieve dyspnea while recumbent (orthopnea); additionally, dyspnea may occur specifically in recumbency on the left side (trepopnea). Paroxysmal nocturnal dyspnea, shortness of breath developing in recumbency, is one of the most highly reliable indicators of HF. Nocturnal cough is a frequently overlooked symptom of HF. These symptoms all typically reflect pulmonary congestion, whereas a history of weight gain, increasing abdominal girth, early satiety, and the onset of edema in dependent organs (extremities or scrotum) indicate right heart congestion; nonspecific, right upper quadrant pain due to congestion of the liver is common in those with significant right-sided HF and may be incorrectly attributed to other conditions. Another cardinal symptom of HF is fatigue, generally held to be reflective of reduction in cardiac output as well as abnormal skeletal muscle metabolic responses to exercise.4 Other causes of fatigue in HF may include major depression, anemia, renal dysfunction, and endocrinologic abnormalities, as well as side effects of medications. Cachexia may be prominent and lead to an extensive workup for malignancy. Information about a patient’s past and current medical problems and a multigenerational family history as well as social history provides the background upon which symptoms are interpreted and a management plan is designed. The presence of hypertension, coronary artery disease and/or diabetes is particularly helpful since these conditions account for approximately 90% of the population attributable risk for HF in the United States.5 The medical history should also focus on what drugs are taken by the patient; notable agents associated with incident HF include cancer chemotherapy,6 diabetes drugs (e.g., thiazolidinediones), ergot-based antimigraine drugs, appetite suppressants, certain antidepressants and antipsychotic agents (notably including clozapine), decongestants such as pseudoephedrine (owing to its ability to trigger severe hypertension), and anti-inflammatory agents such as the antimalarial drug hydroxychloroquine (uncommonly associated with an infiltrative cardiomyopathy) and nonsteroidal anti-inflammatory drugs (NSAIDs). The NSAIDs are well recognized to lead to HF through their ability to worsen renal function, trigger hypertension, and lead to fluid retention, particularly in elderly persons.7 A history of use of herbal remedies and dietary supplements should be obtained. Environmental or toxic exposures including alcohol or drug abuse should be carefully sought. A multigenerational family history should be taken for previous HF or sudden cardiac death. Information about the presence of comorbid conditions (as described later in the chapter) is essential in devising management plans. Although most disorders causing HF are cardiac, it is worth remembering that some systemic illnesses (e.g., anemia, hyperthyroidism) can cause this syndrome without direct cardiac involvement (see Chapter 25). The physical findings listed in Table 23-2 complement information from the medical history in defining the presence and severity of HF (see also Chapter 11). The signs of HF have been extensively described, and much as with the history of patients with HF, components of the physical examination have variable sensitivity and specificity for the diagnosis (see Table 23-4),8 owing in part to the subtlety of some physical findings as well as variability in the physical diagnostic skills of the examiner. No physical finding in HF is absolutely pathognomonic for HFpEF versus HFrEF.9 An evaluation for the presence and severity of HF should include consideration of the patient’s general appearance, measurement of vital signs in the seated and standing positions, examination of the heart and pulses, and assessment of other organs for evidence of congestion or hypoperfusion or indications of comorbid conditions. The patient’s general appearance conveys vital information. The examiner should assess the patient’s body habitus and state of alertness, as well as whether the patient is comfortable, short of breath, coughing, or in pain. The skin examination may show pallor or cyanosis secondary to underperfusion, stigmata of alcohol abuse (such as spider angiomata or palmar erythema), erythema nodosum due to sarcoidosis, bronzing due to hemachromatosis, or easy bruising from amyloidosis; additional findings supporting amyloidosis include deltoid muscle infiltration (leading to the “shoulder pad sign”), tongue hypertrophy, and bilateral thenar wasting from carpal tunnel syndrome. Cheyne-Stokes respiration (also referred to as periodic or cyclic respiration) is common in advanced HF and usually is associated with low cardiac output and sleep-disordered breathing (see also Chapters 25 and 75). The presence of Cheyne-Stokes respiration generally is indicative of an adverse prognosis.10 The details of inspection and palpation of the heart are discussed in Chapter 11. By observing or palpating the apical impulse, the examiner can rapidly determine heart size and quality of the point of maximal impulse. In cases of severe HF, a palpable third heard sound may be present. Cardiac auscultation (Chapter 11) is a crucial part of HF evaluation. The presence of a third heart sound is a crucially important finding and suggests increased ventricular filling volume; although difficult to identify, a third heart sound is highly specific for HF and carries a substantial prognostic meaning. A fourth heart side usually indicates ventricular stiffening. In advanced HF, the third and fourth heart sounds may be superimposed, resulting in a summation gallop. A key objective of the examination in patients with HF is to detect and quantify the presence of volume retention, with or without pulmonary and/or systemic congestion.11 As with symptoms, evidence of congestion does not always indicate with certainty that HF is present, nor does absence of manifest congestion definitively exclude the diagnosis. Patients with HFpEF and those with HFrEF do not generally show significant differences in frequency or significance of the stigmata of volume overload.12 The most definitive method for assessing a patient’s volume status by physical examination is by the measurement of jugular venous pressure (JVP), which is discussed in detail in Chapter 11. An elevated JVP has good sensitivity (70%) and specificity (79%) for elevated left-sided filling pressure.8 The sensitivity and specificity of the JVP in detecting congestion can be considerably improved by exerting pressure on the right upper quadrant of the abdomen while assessing venous pulsations in the neck (hepatojugular reflux). Changes in JVP with therapy usually parallel changes in left-sided filling pressure. Limitations of JVP assessment include difficulties in its evaluation due to body habitus as well as significant interobserver variability in its estimation. Increase in the JVP may lag behind left-sided heart filling pressures or may not rise at all if pulmonary artery pressure is increased to the extent that right ventricular failure or tricuspid insufficiency occur. Conversely, the JVP may be elevated without an increase in left ventricular filling pressures in patients with pulmonary arterial hypertension, in those with isolated right ventricular pressure, or when isolated severe tricuspid regurgitation is present. Although pulmonary congestion is exceedingly common in HF, physical findings indicating its presence are variable, and many are nonspecific. Dullness to percussion and diminished breath sounds at one or both lung bases suggests the presence of a pleural effusion. Bilateral pleural effusions are most common but when an effusion is present unilaterally, it is usually right sided with only approximately 10% occurring exclusively on the left side. Leakage of fluid from pulmonary capillaries into the alveoli can be manifested as rales or rhonchi, and wheezing may occur with reactive bronchoconstriction. Pulmonary rales due to HF usually are fine in nature and extend from the base upwards, whereas those due to other causes (e.g., pulmonary fibrosis) tend to be coarser. Of note, rales or rhonchi may be absent in congested patients with advanced HF; this may reflect compensatory increase in local lymphatic drainage. So-called cardiac asthma is due to the physical presence of fluid in the bronchial wall, as well as secondary bronchospasm,13 and can commonly result in an incorrect diagnosis of obstructive airways disease exacerbation, with consequent mistriage and incorrect therapy with bronchodilators; such incorrect management may be associated with increased risk of death.14 Lower-extremity edema is a common finding in volume-overloaded patients with HF but may commonly be the result of venous insufficiency (particularly after saphenous veins have been harvested for coronary artery bypass grafts) or as a side effect of medications (e.g., calcium channel blockers). Careful inspection of the JVP will help improve the specificity of pedal edema for HF. Detection of reduced cardiac output and systemic hypoperfusion is a key component of the examination. Although patients with poor systemic perfusion usually have low systolic and narrow pulse pressures as well as weak and thready pulses, this relationship is not exact. Many patients with systolic blood pressures in the range of 80 mm Hg (or even lower) may have adequate perfusion, whereas others with reduced cardiac output may maintain blood pressure in the normal range at the expense of tissue perfusion by greatly increasing systemic vascular resistance. Findings suggesting reduced cardiac output include poor mentation, reduced urine output, mottled skin, and cool extremities. Of these, cool extremities are the most broadly useful. Assessment for systemic congestion, taken together with evaluation for reduced cardiac output, may be useful to separate patients with HF (Fig. 23-2) into “dry/warm” (uncongested with normal perfusion), “wet/warm” (congested with normal perfusion, the most common combination found in decompensated HF), “dry/cold” (uncongested but hypoperfused), and “wet/cold” (cardiogenic shock) categories,15 as discussed in Chapter 24. A suggested algorithm for the diagnostic evaluation of HF is presented in Figure 23-1. The laboratory testing and imaging modalities described next provide important information for the diagnosis and management of patients with suspected or proven HF. Despite advances in other imaging technologies, plain chest radiography (see also Chapter 15) remains a very useful component of the assessment, particularly when the clinical presentation is ambiguous. Results of chest radiography are additive to clinical variables from history and physical examination, and similarly complement the results of biomarker testing. Accordingly, chest radiography should be a routine part of the early evaluation of patients presenting with symptoms suggestive of acutely decompensated HF. The electrocardiogram (ECG) (see also Chapter 12) is a standard part of the initial evaluation of a patient with suspected HF, because it may provide important clues regarding incident HF, while also assisting in investigation of episodes of decompensation in previously diagnosed patients. In patients with HF, the ECG infrequently is normal but may show only nonspecific changes; thus, much as with chest radiography, the positive predictive value of ECG far surpasses its negative predictive value in this setting. Patients with new-onset HF and those with acute decompensation of chronic HF should undergo testing with a laboratory panel that includes electrolytes, blood urea nitrogen, serum creatinine, hepatic enzymes, fasting lipid profile, thyroid-stimulating hormone, transferrin saturation, uric acid, a complete blood count, and a urinalysis. As discussed further on, the natriuretic peptides may be exceedingly useful for diagnosis as well as for prognostication. A test for human immunodeficiency virus infection or further screening for hemachromatosis is reasonable in selected patients, whereas diagnostic tests for rheumatologic diseases, amyloidosis, or pheochromocytoma are reasonable when suspicion exists for these diseases.
Clinical Assessment of Heart Failure
Heart Failure Definitions
Symptoms and Signs Associated with HF
Historical Information Helpful in Determining if Symptoms Are Due to HF


The Medical History and Physical Examination
Heart Failure Symptoms
FINDING
FREQUENCY
SENSITIVITY
SPECIFICITY
PREDICTIVE VALUE
LR
OR (95% CI)
Positive
Negative
Positive
Negative
Rales (heard over ≥1/3 lung fields)
26/192
15
89
69
38
1.32
1.04
1.4 (0.6, 3.4)
S3
123/192
62
32
61
33
0.92
0.85
0.8 (0.4, 1.5)
Ascites (moderate/massive)
31/192
21
92
81
40
2.44
1.15
2.8 (1.1, 7.3)
Edema (≥2+)
73/192
41
66
67
40
1.20
1.11
1.3 (0.7, 2.5)
Orthopnea (requiring ≥2 pillows)
157/192
86
25
66
51
1.15
1.80
2.1 (1, 4.4)
Hepatomegaly (liver edge palpable at >4 fingerbreadths beyond costal margin)
23/191
15
93
78
39
2.13
1.09
2.3 (0.8, 6.6)
Hepatojugular reflux
147/186
83
27
65
49
1.13
1.54
1.7 (0.9, 3.5)
JVP ≥12 mm Hg
101/186
65
64
75
52
1.79
1.82
3.3 (1.8, 6.1)
JVP <8 mm Hg
18/186
4.3
81
28
33
0.23
0.85
0.2
Other Historical Information
The Physical Examination
Routine Assessment
Chest Radiography
The Electrocardiogram
Measurement of Blood Chemistry and Hematologic Variables
Clinical Assessment of Heart Failure
23
FIGURE 23-1 Flow chart for the evaluation of patients with HF. Appropriate criteria for natriuretic peptide testing (*) to identify or exclude HF are summarized in Table 23-6. The diagnosis of HF is made using a combination of clinical judgment and initial and subsequent testing. After a thorough history and physical examination, along with initial diagnostic testing, imaging (such as with echocardiography) may still be necessary in ambiguous cases to definitively identify or exclude HF. Cut-off values for BNP and NT-proBNP are displayed in Table 23-6. ECG = electrocardiogram.