Classification and Terminology of Cardiovascular Anomalies
Joseph J. Maleszewski
William D. Edwards
Perspectives on Nomenclature
Through 5,000 years of recorded human history, only during the past 60 have treatments become available to substantially improve the quality of life and increase the longevity of children with cardiac anomalies. Within these 60 years, diagnostic and interventional procedures have been developed that have defined the frontiers of medical technology and creativity. During these exciting and innovative times, however, seeds were also sown in the form of redundant and overlapping terminology, that have inadvertently led to difficulty and confusion for those interested in the subject of congenital heart disease.
Diversity of Terminology
Drawings and descriptions of malformed hearts date back to the 18th century, and in the mid-19th century Peacock published 18 cases in his classic series (1). However, it is the categorization of 1,000 malformed hearts by Dr. Maude E. Abbott at McGill University, published by the American Heart Association in 1936 which stands as a landmark in the classification of congenital heart disease (2). Since that time, others have examined large numbers of cases, both from autopsies and from living patients, and have proposed different systems of classification and nomenclature (3,4,5,6,7,8,9,10,11,12,13). Furthermore, numerous classifications have been adopted for individual anomalies, such as ventricular septal defects, and for groups of anomalies, such as hearts with univentricular atrioventricular connections.
Each new system, however, has reflected not only the state of knowledge at the time of its formulation, but also the particular interests or biases of its creators. Thus, among the various classifications, some researchers have emphasized surgical anatomy, some embryogenesis, others spatial relationships such as atrial sidedness and the positions of ventricles and great arteries, and still others clinical features such as cyanosis and altered pulmonary blood flow. Not surprisingly, the introduction of new systems of classification has been attended by changes in terminology, primarily to clarify or simplify certain concepts. But as newer terms have been introduced, older terms have rarely been abandoned. As a result, the nomenclature for congenital heart disease has become a sea of synonyms, adding confusion rather than clarity to an already complex subject (Appendix 7.1).
Unity from Diversity
Is the development of a unified system of nomenclature a realistic and worthwhile goal? If so, who should decide on the acceptable terminology? And what price will be paid to gain unity at the expense of diversity? Although such a system would limit the confusing number of synonyms that now exist, it could also limit our perspective as certain terms (such as those with an embryologic basis) are purged from our rich and diverse heritage.
Nevertheless, a movement is already well underway to establish such a unified system. Uniform acceptance, however, will probably be achieved only following the publication of a consensus report from an international group that represents all disciplines dealing with congenital heart disease. To date, such attempts have met with only limited success (14). The system that is eventually chosen should be accurately descriptive, internally consistent, clinically usable, readily codable in a database, and applicable to all forms of congenital heart disease. New terms should be accepted only if they are necessary, brief, and specific (3).
Until such a consensus report is available, it is premature to endorse a specific system of nomenclature. However, at least at an institutional level, those dealing with patients having congenital heart disease should agree to speak the same clinical language and to prescribe to a single system of terminology. For this chapter, the approach and terminology suggested by Anderson et al. (4) are emphasized, with some modifications. Commonly used Latin terms and their anglicized counterparts are provided in Appendix 7.2.
Although the notation system proposed by Van Praagh (3) is not emphasized in this chapter, it is favored at some institutions and warrants summarization. The situs (sidedness) is determined separately for the atria, ventricles, and great arteries. Atrial situs may be solitus (S), inversus (I), or ambiguous (A). Ventricular situs is solitus or D-loop (D), inversus (I) or L-loop (L), or ambiguous (X). Great arterial situs is designated as solitus (S), inversus (I), D-transposition/malposition (D), L-transposition/malposition (L), or ambiguous or anterior transposition/malposition (A). Abbreviations are listed within the parentheses, with the ventriculoarterial arrangement included before the parentheses and any atrioventricular malalignments or other anomalies stated after the parentheses. Thus, TGA {S, D, D} would correspond to complete transposition of the great arteries.
Sequential Segmental Analysis
For evaluating patients with suspected congenital heart disease, it is helpful to consider the heart as a segmented structure represented by three regions—atria, ventricles, and great arteries (3,4,5,6). Each region, in turn, is partitioned into two components, usually right-sided and left-sided. Atrioventricular valves serve as connectors between atria and ventricles, and semilunar valves join the ventricles to the great arteries. There is only a limited number of possible connections between the three major regions, regardless of their spatial orientations. In practice, each region is evaluated independently, following the direction of blood flow: (a) systemic and pulmonary veins, (b) atria, (c) atrioventricular valves, (d) ventricles and right ventricular outflow tract (infundibulum or conus), (e) semilunar valves, and (f) great arteries.
In a systematic manner, right-sided and left-sided structures at each level are evaluated according to their morphology; their relative positions; their connections to proximal and distal segments; and the presence and location of shunts, obstructions, and valvular regurgitation (7). This constitutes the sequential segmental method
for the investigation of congenital heart disease, and represents a diagnostic cornerstone both for clinicians and for pathologists. Before applying this method, however, it is important to determine the cardiac position and the visceral situs (sidedness).
for the investigation of congenital heart disease, and represents a diagnostic cornerstone both for clinicians and for pathologists. Before applying this method, however, it is important to determine the cardiac position and the visceral situs (sidedness).
Cardiac Position and Apical Direction
With regard to the position of the heart in the chest, two questions arise that can be answered independently: Where is the heart located, and what is the direction of the cardiac apex? Unfortunately, the terms levocardia, dextrocardia, and mesocardia are commonly used to answer both questions, thus imparting an element of ambiguity (3,4). Although the approach described below is not universally accepted, it does provide clarity by defining the cardiac location and apical direction separately and by avoiding the ambiguous Latin terms.
Location in the Chest
Within the thorax, the heart can be described positionally as left-sided (normal), right-sided, or midline. This designation is particularly useful radiographically, before a patient has been evaluated by other imaging techniques.
The position of the heart in the mediastinum is affected not only by underlying cardiac malformations but also by abnormalities in adjacent structures. It can be displaced by conditions that distort the shape of the thorax, such as severe scoliosis or an elevated diaphragm, or that alter the size of thoracic structures, such as a hypoplastic lung or diaphragmatic hernia. Rightward displacement of the heart constitutes dextroposition, a leftward shift represents levoposition, and shifts toward the midline are called mesoposition.
In rare instances, sternal or diaphragmatic defects exist and are associated with an extrathoracic heart, or ectopia cordis (ectopic heart). This condition may be partial or complete and can be further categorized as cervical, thoracocervical, thoracic, thoracoabdominal, or abdominal.
Orientation in the Chest
The direction in which the ventricles are aligned defines the base–apex axis of the heart and may be leftward, rightward, or midline (Figs. 7.1 and 7.2). Leftward ventricles represent the normal state and are characterized by an apex that is directed leftward, anteriorly, and somewhat inferiorly. The extent of these three directions is variable and is influenced by age, body build, and the level and functional state of the diaphragm. Ventricles with a rightward apex are directed to the right of midline. In contrast, midline ventricles are often box-shaped and exhibit two apices that are directed anteriorly and inferiorly (7).
The base–apex axis is independent of cardiac location and displacements. For example, a patient with a hypoplastic right lung could have a right-sided heart, owing to dextroposition, and still exhibit a leftward apex (Fig. 7.1B).
The base–apex axis is also independent of cardiac sidedness. Thus, the presence of a leftward apex does not necessarily imply normal sidedness (situs solitus), and a right-sided apex does not always coincide with mirror-image sidedness (situs inversus). A midline apex, on the other hand, usually is associated with cardiac isomerism (situs ambiguus).
Visceral Sidedness (Situs)
All major organ systems begin their embryologic development as midline structures with bilateral mirror-image symmetry. However, three organ systems (cardiovascular, respiratory, and digestive) later acquire asymmetry and are thereby characterized by sidedness (situs or handedness), which is genetically determined. Sidedness may be normal, mirror-image, isomeric, or indeterminate. Right isomerism indicates bilateral right-sidedness, whereas left isomerism denotes bilateral left-sidedness.
Isomerism and Splenic Anomalies
The relationship between isomerism and splenic anomalies is intriguing (8). The splenic anlage, rather than originating as a midline structure, appears to be left-sided from its inception. Thus, when right isomerism exists, the spleen is usually absent (asplenia syndrome). Left isomerism, in contrast, is generally associated with multiple spleens (polysplenia syndrome) that are confined to only one side of the vertebral column. Occasionally, subjects with asplenia or polysplenia have normal hearts, and, rarely, those with atrial isomerism have normal spleens.
Abnormalities may affect the entire body, as in total mirror images sidedness (situs inversus totalis), or can involve individual organ systems. Moreover, sidedness may vary between systems,
particularly in conditions associated with isomerism. Although the term atriovisceral situs enjoys common usage, it does not always allow an accurate description of sidedness in the asplenia and polysplenia syndromes. Consequently, it is recommended that the sidedness of cardiovascular, respiratory, and digestive systems be designated separately (7).
particularly in conditions associated with isomerism. Although the term atriovisceral situs enjoys common usage, it does not always allow an accurate description of sidedness in the asplenia and polysplenia syndromes. Consequently, it is recommended that the sidedness of cardiovascular, respiratory, and digestive systems be designated separately (7).
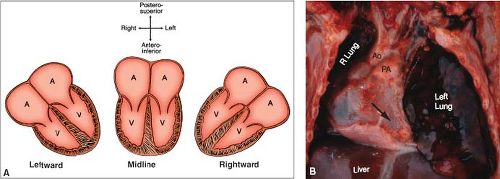 Figure 7.1 Cardiac base–apex axis. A: The three types are shown schematically and are independent of cardiac position or situs. B: The ventricular apex is leftward (arrow), even though a hypoplastic right lung has caused dextroposition of the entire heart. (See Appendix 6.1 for abbreviations.) |
Cardiac Sidedness (Situs)
Cardiac sidedness is determined by the position of the morphologic right
atrium (Fig. 7.3). It is not determined by the direction of the cardiac apex, the positions of the ventricles or great arteries, or the sidedness of noncardiac viscera. The morphologic right atrium is normally right-sided but is left-sided in situs inversus (mirror-image sidedness). Bilateral right atria define right cardiac isomerism, and bilateral left atria constitute left cardiac isomerism (Fig. 7.4) (14). In some cases of polysplenia, one chamber represents a left atrium, but the other has a hybrid appearance that is morphologically neither left nor right; this constitutes indeterminate cardiac sidedness. In practice, an accurate determination of cardiac sidedness depends on an accurate distinction between right and left atrial morphology, as discussed in this chapter and in Chapter 6. Although all investigators agree on the concept of normal and mirror-image cardiac sidedness, some have questioned the existence of atrial isomerism (9).
atrium (Fig. 7.3). It is not determined by the direction of the cardiac apex, the positions of the ventricles or great arteries, or the sidedness of noncardiac viscera. The morphologic right atrium is normally right-sided but is left-sided in situs inversus (mirror-image sidedness). Bilateral right atria define right cardiac isomerism, and bilateral left atria constitute left cardiac isomerism (Fig. 7.4) (14). In some cases of polysplenia, one chamber represents a left atrium, but the other has a hybrid appearance that is morphologically neither left nor right; this constitutes indeterminate cardiac sidedness. In practice, an accurate determination of cardiac sidedness depends on an accurate distinction between right and left atrial morphology, as discussed in this chapter and in Chapter 6. Although all investigators agree on the concept of normal and mirror-image cardiac sidedness, some have questioned the existence of atrial isomerism (9).
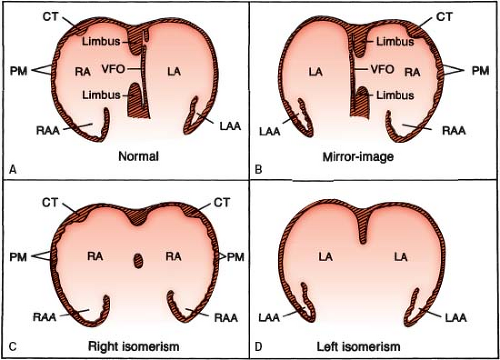 Figure 7.3 Cardiac situs (sidedness), shown schematically. A: Situs solitus, with right-sided morphologic right atrium. B: Situs inversus, with left-sided morphologic right atrium. C, D: Situs ambiguus, with bilateral morphologic right atria (C) and bilateral morphologic left atria (D). (See Appendix 6.1 for abbreviations.) |
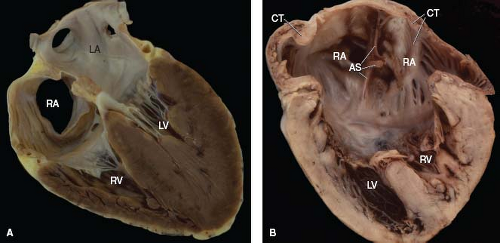 Figure 7.4 Cardiac situs in two specimens, displayed in the four-chamber format. A: Situs solitus (normal cardiac sidedness). B: Situs ambiguus, with right isomerism. A crista terminalis is present bilaterally. Ventricular inversion is also present but plays no role in determining cardiac sidedness. (See Appendix 6.1 for abbreviations.) |
Pulmonary Sidedness (Situs)
Pulmonary sidedness is determined by the positions of the morphologic right and left lungs (Fig. 7.5). Pulmonary morphology, in turn, is defined by the relationship of the pulmonary arteries to their adjacent bronchi, and not by the number of lobes.
The pulmonary artery of a morphologic right lung travels anterior to its upper and intermediate bronchi, whereas that of a morphologic left lung travels superior to its main bronchus and posterior to the upper lobe bronchus.
The pulmonary artery of a morphologic right lung travels anterior to its upper and intermediate bronchi, whereas that of a morphologic left lung travels superior to its main bronchus and posterior to the upper lobe bronchus.
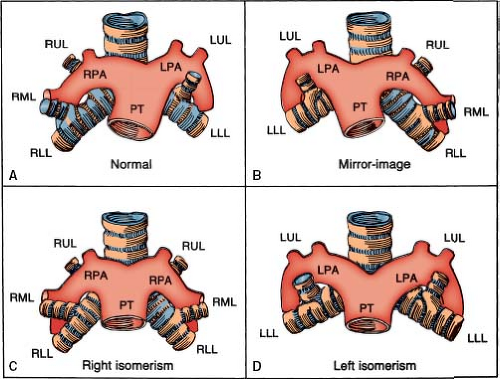 Figure 7.5 Pulmonary situs (sidedness), shown schematically. A: Situs solitus, with right pulmonary anterior to its upper lobe bronchus and with left pulmonary artery posterior to its upper lobe bronchus. B: Situs inversus, with mirror-image morphology. C, D: Situs ambiguus, with bilateral morphologic right lungs (C) and bilateral morphologic left lungs (D). (See Appendix 6.1 for abbreviations.) |
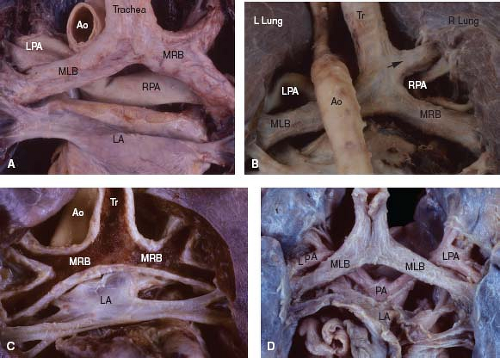 Figure 7.6 Pulmonary situs in four specimens, viewed posteriorly. A: Normal situs, with long left bronchus and short right bronchus. B: Normal situs, with tracheal origin of the right upper lobe bronchus (bronchus suis) (arrow). C: Right pulmonary isomerism, with short bronchi of similar length (the posterior wall of the airways has been removed). D: Left pulmonary isomerism, with long bronchi of similar length. (See Appendix 6.1 for abbreviations.) |
Clinically, pulmonary sidedness may be inferred by comparing the relative lengths of the two main bronchi, as measured on a chest radiograph that shows an air bronchogram. Normally, the distance from the carina to the origin of the upper lobe bronchus is 1.5 to 2.5 times greater for the morphologic left lung than for the right lung, and this ratio holds true regardless of the sidedness of the aortic arch (which is determined by the bronchus over which the aorta travels) (10). With pulmonary isomerism, this ratio approaches unity because the lengths of the two mirror-image bronchi are similar (Fig. 7.6).
Although most examples of bilateral trilobed lungs do correspond to cases of right isomerism or asplenia syndrome, bilateral bilobed lungs more often occur as a variation of normal morphology than as a manifestation of left isomerism or polysplenia.
Abdominal Sidedness (Situs)
Abdominal sidedness is determined by the location of the liver and stomach, with the spleen and pancreas generally on the same side of the vertebral column as the stomach (Fig. 7.7). The state of the spleen can be evaluated by clinical imaging, thus supplanting the less reliable method of identifying Howell–Jolly bodies in peripheral blood smears. At autopsy, splenic morphology should always be designated in patients with congenital heart disease.
In the asplenia syndrome, the liver is commonly midline with two mirror-image right lobes (right hepatic isomerism). The biliary tree
is patent and is usually associated with a single gallbladder (Fig. 7.8). The position of the stomach and pancreas can be left-sided, right-sided, or midline. Malrotation of the bowel is the rule, such that the cecum and appendix may be located in any of the abdominal quadrants. Finally, the aorta and inferior vena cava travel together on the same side of the vertebral column, a unique feature that can be demonstrated by abdominal imaging.
is patent and is usually associated with a single gallbladder (Fig. 7.8). The position of the stomach and pancreas can be left-sided, right-sided, or midline. Malrotation of the bowel is the rule, such that the cecum and appendix may be located in any of the abdominal quadrants. Finally, the aorta and inferior vena cava travel together on the same side of the vertebral column, a unique feature that can be demonstrated by abdominal imaging.
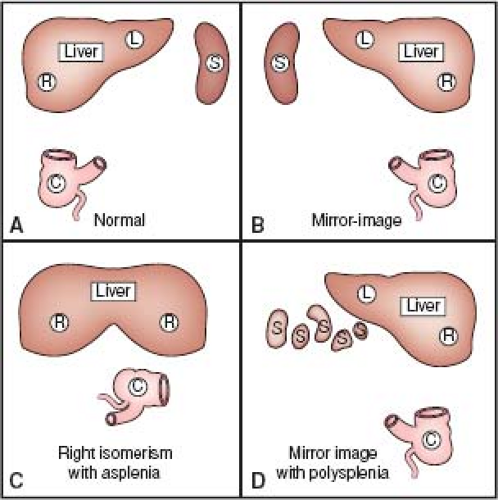 Figure 7.7 Abdominal situs (sidedness), drawn schematically. A: Situs solitus, with right-sided liver, left-sided spleen (also stomach and pancreas, not shown), and cecum in right lower quadrant. B: Situs inversus, with mirror-image morphology. C: Situs ambiguus with right isomerism shows liver with two right lobes, malrotation of the bowel (indicated by the abnormal position of the cecum), and asplenia. D: Situs ambiguus with left isomerism shows polysplenia; other features are variable. (See Appendix 6.1 for abbreviations.) |
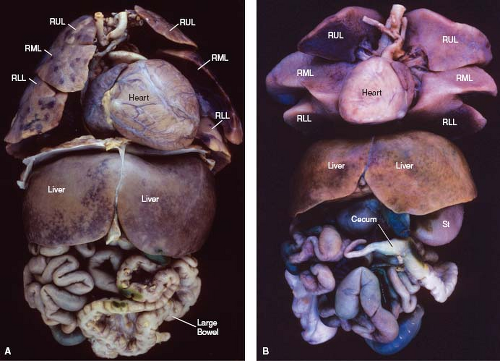 Figure 7.8 Abdominal situs in two patients, with thoracoabdominal organs viewed anteriorly. A, B: Both patients had the asplenia syndrome, with right isomerism of the heart, lungs, and abdominal organs. Both exhibit a midline symmetric liver and malrotation of the bowel (with the small bowel to the right and the large bowel to the left). (As an aside, cardiac situs is not affected by the cardiac base–apex axis, which is leftward in A and rightward in B.) (See Appendix 6.1 for abbreviations.) |
Interestingly, with the polysplenia syndrome, the sidedness of the abdominal viscera may be indeterminate (ambiguous), mirror-image (inversus), or even normal (solitus). Although the spleens are multiple, they are all located on the same side of the vertebral column as the stomach. The gallbladder is single, but biliary atresia may occur. As a rule, the inferior vena cava fails to join the heart directly and exhibits azygos continuation, with connection to the superior vena cava.
Morphology of Cardiac Segments
Accurate identification of right-sided and left-sided structures is an essential feature of the sequential segmental approach to the diagnosis of congenital heart disease. For consistency and reproducibility of diagnoses, definitions of even the most commonly used terms are helpful.
In this regard, a distinction between the terms connection and drainage is necessary because the two are not synonymous. Connection is an anatomic term that implies a direct link between two structures. In contrast, drainage is a hemodynamic term that refers to the direction of blood flow.
A distinction also should be drawn between single and common, as applied to cardiac chambers and valves. Single implies that the corresponding contralateral structure is entirely absent. Tricuspid atresia with a single-inlet ventricle is one example. In contrast, the term common indicates bilateral components with absent septation. Examples are a common atrium, a common atrioventricular valve, and a common truncal artery (truncus arteriosus).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree