Survival rate of newborns. Survival rate of 34,650 newborns after reported ECMO to the Extracorporeal Life Support Organization (ELSO), grouped by cause of admission to ECMO (ERCP Extracorporeal cardiopulmonary resuscitation)
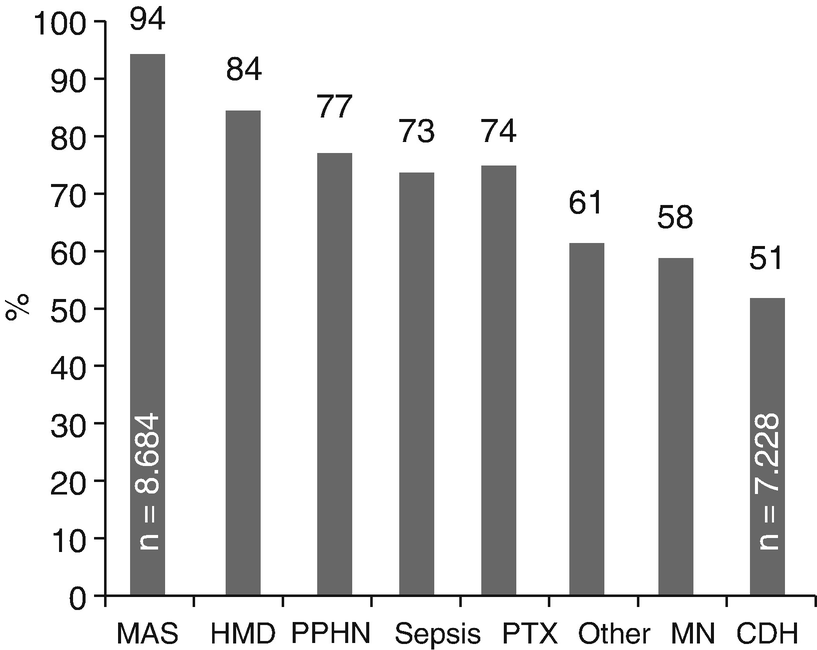
Survival rate of Nnwborns. Survival rate to discharge of 27,728 newborns treated with ECMO, reported to ELSO by respiratory condition (MAS Meconium aspiration syndrome, HMD Hyaline membrane disease, PPHN Persistent pulmonary hypertension of the newborn, PTX Pneumothorax, PN Pneumonia, CDH Congenital diaphragmatic hernia)
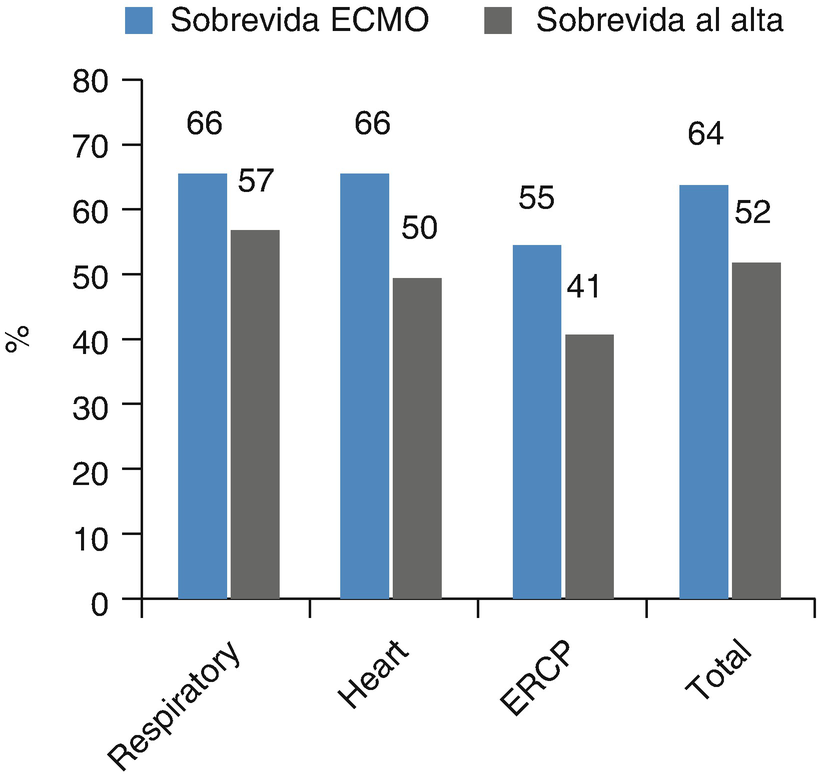
Survival rate of pediatric patients. Survival rate of 16,253 pediatric patients after ECMO reported to the Extracorporeal Life Support Organization (ELSO), grouped by cause of admission to ECMO (ERCP Extracorporeal cardiopulmonary resuscitation)
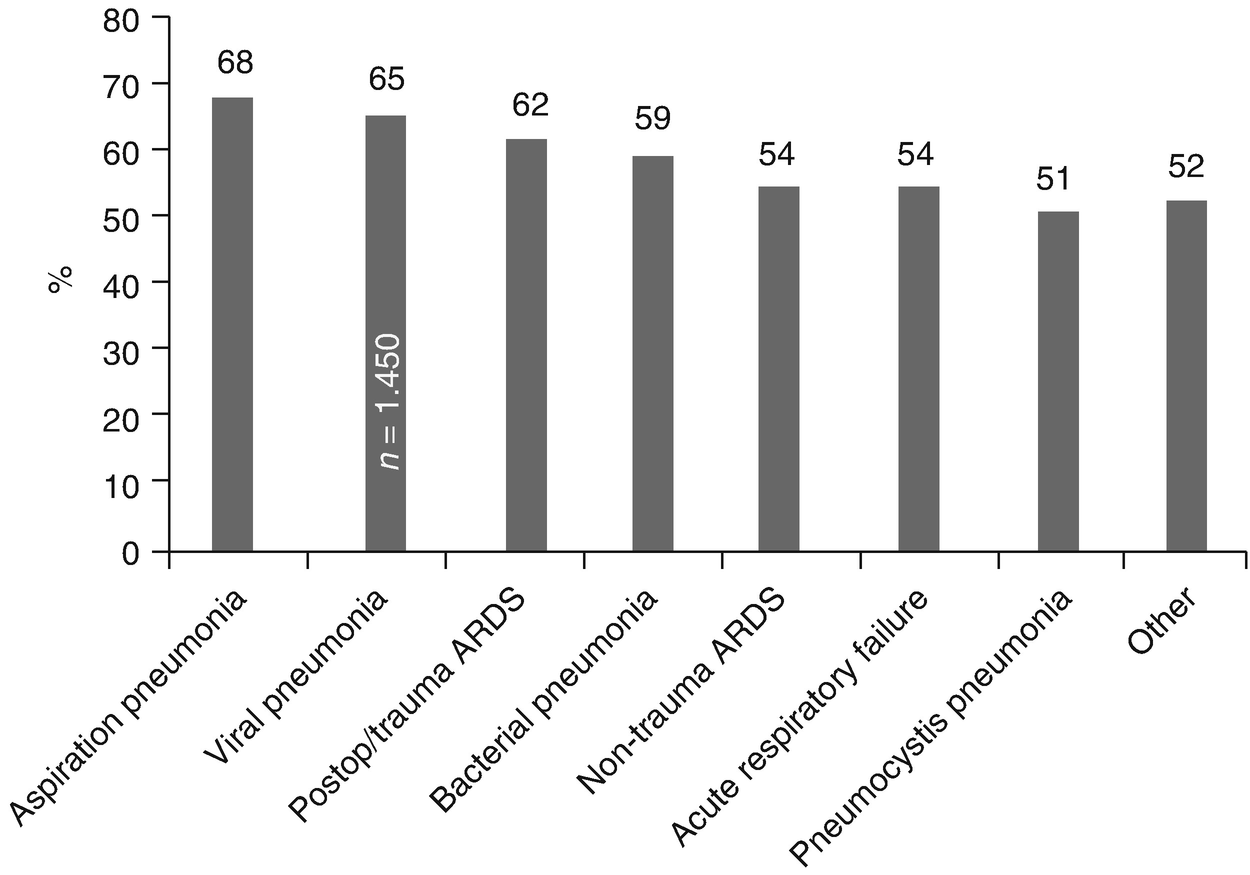
Survival rate of pediatric patients. Survival rate of 6569 discharged pediatric patients treated with ECMO reported to ELSO by respiratory condition (ARDS Acute respiratory distress syndrome)
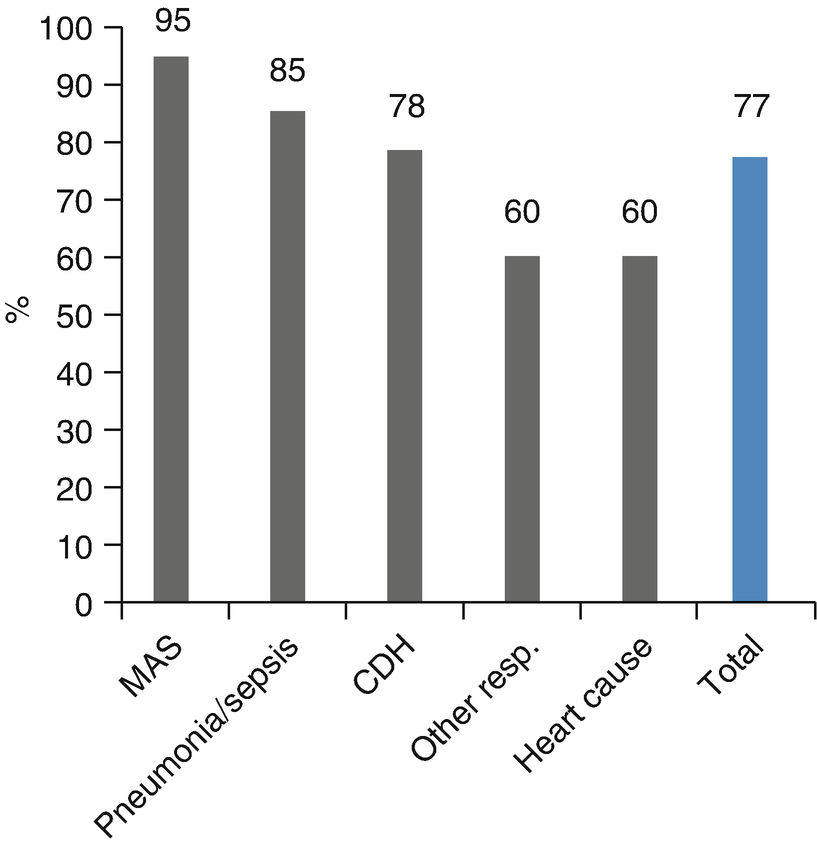
Survival rate to discharge. Survival rate to discharge of 143 patients (123 newborns and 20 pediatric patients) treated in the Newborn-Pediatric ECMO Program at the Clinical Hospital of the Pontificia Universidad Católica de Chile (ECMO-UC) 2003–2014, reported to ELSO by main diagnosis (MAS Meconium aspiration syndrome; CDH Congenital diaphragmatic hernia)
In 1972, Bartlett reported the first case of successful prolonged post-operative cardiac support in a 2-year-old patient who suffered from post-surgery heart failure after a Mustard procedure because of transposition of great arteries. At present, more than half of patients requiring perioperative heart ECMO are those suffering from complex cyanotic congenital heart diseases. The largest group of patients requiring ECMO support is those who, after cardiotomy, present complete AV canal (20%), complex single ventricle anomaly (17%), and tetralogy of Fallot (14%). Among the chief causes requiring perioperatory heart ECMO are hypoxia (36%), cardiac arrest (24%), and failure after leaving extracorporeal circulation support (14%).
ECMO Physiology
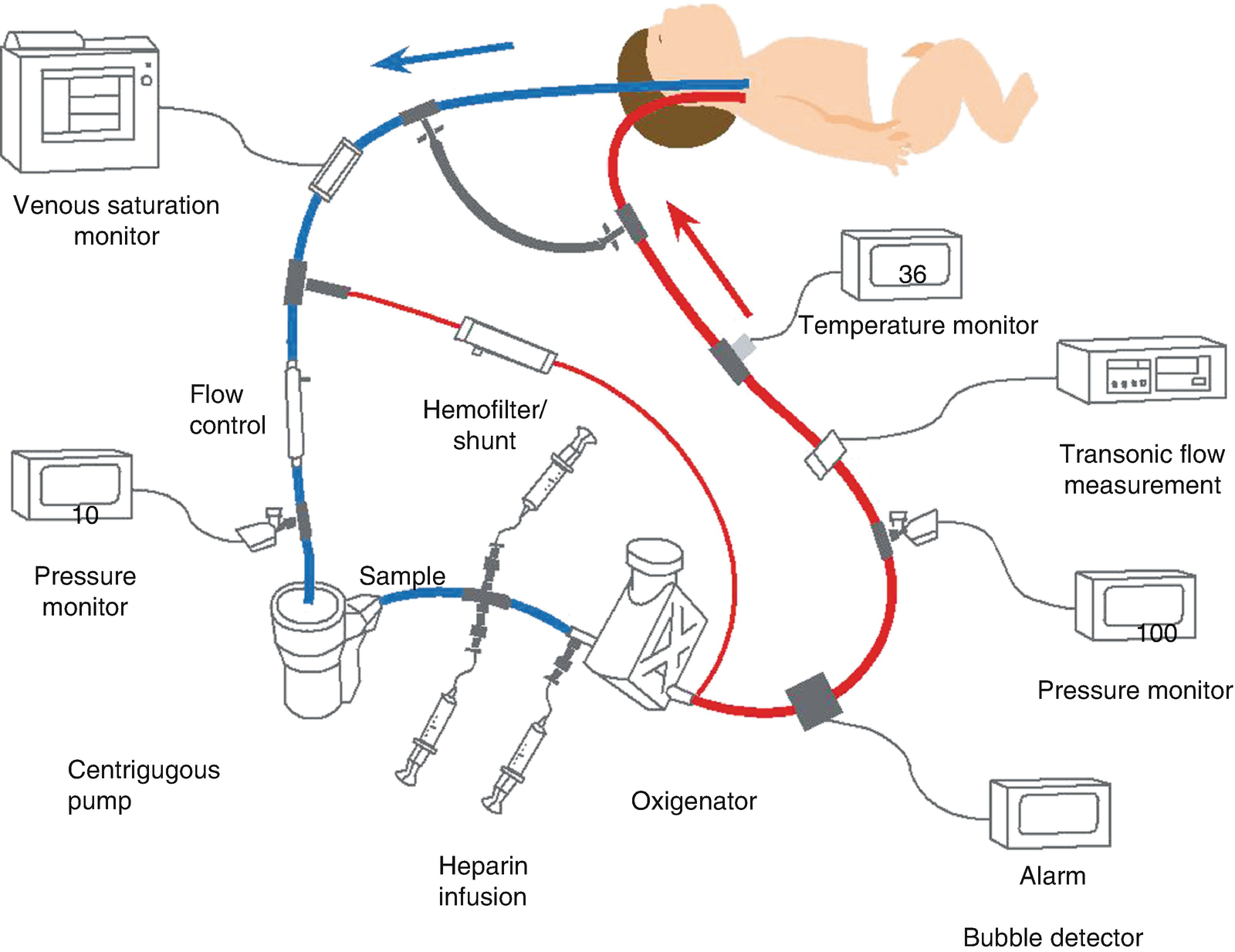
Diagram of veno-arterial ECMO with pump and oxygenator. Venous blood is obtained from the right atrium through the right internal jugular vein. It is then pumped, oxygenized, heated and returned to the aorta through the right carotid artery. (Diagram used with permission from ECMO Manual of the Children’s National Medical Center, George Washington University, Washington DC, 2010)
There are in principle two different forms of ECMO:
- (a)
Veno-arterial (VA): Blood is drained from the right atrium through a cannula inserted into the right internal jugular vein, the femoral vein, or directly to the right atrium; and it is picked back up at the thoracic aorta through a right carotid cannula, a femoral cannula or an aortic cannula. VA ECMO provides heart-lung support. It is common to use a transthoracic cannula (right ventricle and aortic cannula) in patients who have endured heart operations.
- (b)
Veno-venous (VV): Blood is drained from the right atrium through the posterior and inferior orifices of a double-lumen cannula inserted into the right jugular vein and returned to the same right ventricle through the anterior orifices of the cannula, which are pointed toward the tricuspid valve. One of the limits of this method lies in the recirculation of already oxygenized blood through the double lumen cannula. This has been corrected with the new design for VV cannulas. VV ECMO may also be performed in older children through the use of two cannulas, draining blood from the jugular vein and returning it through the femoral vein. VV ECMO requires a well-functioning heart. This modality of ECMO prevents cannulation in the carotid or femoral arteries, thus reducing complications arising from cannulation or ligation of these arteries, as well as those arising from air entering the ECMO circuit. This method has seen an increase in usage during recent years, covering 40% and 50% of respiratory cases in newborn and pediatric patients, respectively. Oxygen is delivered during ECMO by the combination of blood oxygenation through the membrane, blood flowing through the extracorporeal circuit, native lung oxygenation, and native heart output. In turn, oxygenation at the ECMO membrane is a function of its geometry, material composition and thickness, blood and FiO2 laminar thickness, time of permanence of red blood cells in the exchange area, hemoglobin concentration, and O2 saturation. On the other hand, CO2 removal during ECMO is a function of the geometry, materials, and surface area of the membrane, blood PCO2 and, to a lesser extent, it depends on blood and gas flows through the membrane.
In a VA ECMO, the bypass generates an essentially non-pulsatile blood flow. In this way, as the blood flow to the extracorporeal circuit increases, the pulse wave decreases, completely ceasing when it reaches a 100% bypass, except for occasional waves. However, it is normal for VA ECMO to only involve about an 80% bypass, allowing a blood flow of 20% or more through the left heart and lungs, resulting in a reduced but visible pulse wave. The kidney is without doubt the most affected organ by the absence of pulsatility, producing an antidiuretic effect because of juxtaglomerular stimulation. In addition, non-pulsatile flow has been linked to stimulation of the pressure receptors in the carotid sinus, causing a large release of catecholamines, with damaging effects to microcirculation.
Selection Criteria for Applying ECMO
Selection criteria for newborns
Gestational age ≥34 weeks |
Weight at birth ≥2000 grams |
Unresponsive to maximum medical care (including HFOV, iNO, surfactant) |
Reversible cardiopulmonary condition |
Mechanical ventilation ≤14 days |
High pulmonary mortality (50–100%), considering: |
Oxygenation index (OI) >35–40 for 4–6 hours (iNO, HFOV) |
PaO2 < 40 mmHg for 4 h (100% O2) |
OI ≥ 25 after 72 h with HFOV-iNO |
Unmanageable metabolic acidosis (ph < 7.15 for 2 h) |
Reduced cardiac output with reversible etiology |
Impossibility to wean from cardiopulmonary bypass |
As a bridge for heart transplant |
No untreatable congenital cardiopathy or injuries after heart surgery |
Absence of major intracranial hemorrhage (≥ III degree) |
Absence of uncontrollable hemorrhage |
No evidence of irreversible brain damage |
No malformations or genetic syndromes with fatal prognosis |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


