Ciliary structure
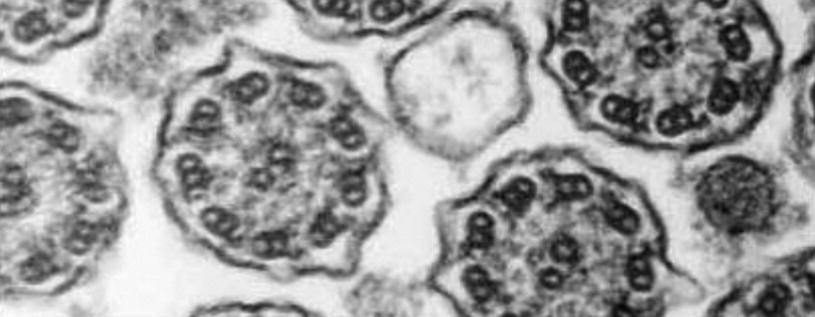
Normal cilia. Cross section of normal cilium and primary ciliary dyskinesia (PCD). Uranyl acetate and lead citrate. ×100,000
Ciliary Function
A cylindrical pseudostratified mucosa covers the respiratory epithelium, which ensures mucociliary transport. This structure is one of the most important first-line defenses of the airway, for both the lower tract and upper tract, including the paranasal sinus and middle ear. Thus, the entrance of particles and infection agents is avoided, protecting the person. This function is the so-called mucociliary clearance . Impairment of this system may cause infections in the respiratory tract.
The movement of the microtubule doublets determines the ciliary beat. The external arm of dynein controls ciliary frequency, and the internal arm of the dynein directs the shape of the wave. Radial spokes, which are related to the central pair of microtubules, are in charge of activating the dynein arms. The central pair/radial spoke complex is the regulating key for the activation of the dynein arms.
Cilia have a rhythmic beat. Each cycle has two active components: one movement during which the cilium is completely extended perpendicularly relative to the cellular surface, and a recovery movement in which the inclined cilium moves parallel to the cellular surface, toward its original position. The duration of this phase depends on the frequency of the ciliary beating.
Cilia in adjacent cells are coordinated to beat simultaneously and at the same time. In this way, an effective cilia motility is caused, which expels the mucus. The frequency of the ciliary beat oscillates between 13 and 27 Hz in the respiratory mucosa. It is known that multiple factors affect ciliary motility, such as neural control, external factors (temperature, humidity), and pharmacological effects.
To have an efficient motility and effective mucociliary clearance, it is crucial to conserve the ciliary structure intact, besides keeping an adequate amount and quality of secretions that allow good movement, as well as a good synchronization in the ciliary motility with a correct frequency of ciliary beat.
Age has no impact on the frequency of ciliary beat, which is similar for patients between 3 months old and 74 years old.
Ciliary function can be affected by external factors, such as bacterial toxins and inflammation mediators, which cause ultrastructural cilium alterations and ciliary dysfunction.
Alterations in Ciliary Structure
The ciliary structure may sustain different types of morphological alterations, such as the absence of internal and external dynein arms, alteration of the radial spokes, and cilium fusion.
Anomalies of the cilium ultrastructure may be divided into primary defects, which are those observed in PCD, and secondary defects, which are caused by infections, cigarette smoke, or pollutants. These secondary alterations are different from those found in PCD, and they consist mainly of defects in the microtubules and composed cilia, affecting up to 10% of cilia in normal patients, and when challenged by severe respiratory infections, up to 17% of the cilia may be affected.
Primary ciliary dyskinesia (PCD) structural abnormalities
A. Dynein arms |
Absence or reduction of the number of internal and external arms Absence or reduction of the number of internal arms Absence or reduction of the number of external arms |
B. Radial spokes |
Absence |
C. Microtubules |
Defects on the peripheral microtubules Absence of the central pair with the transposition of a peripheral pair to the center |
D. Complete ciliary aplasia |
E. Orientation defect |
In one phenotype of PCD, the ultrastructure is normal, but the cilia are not normally oriented in relation to other cilia. This orientation disorder may be primary, but it may as well occur secondary to an infection.
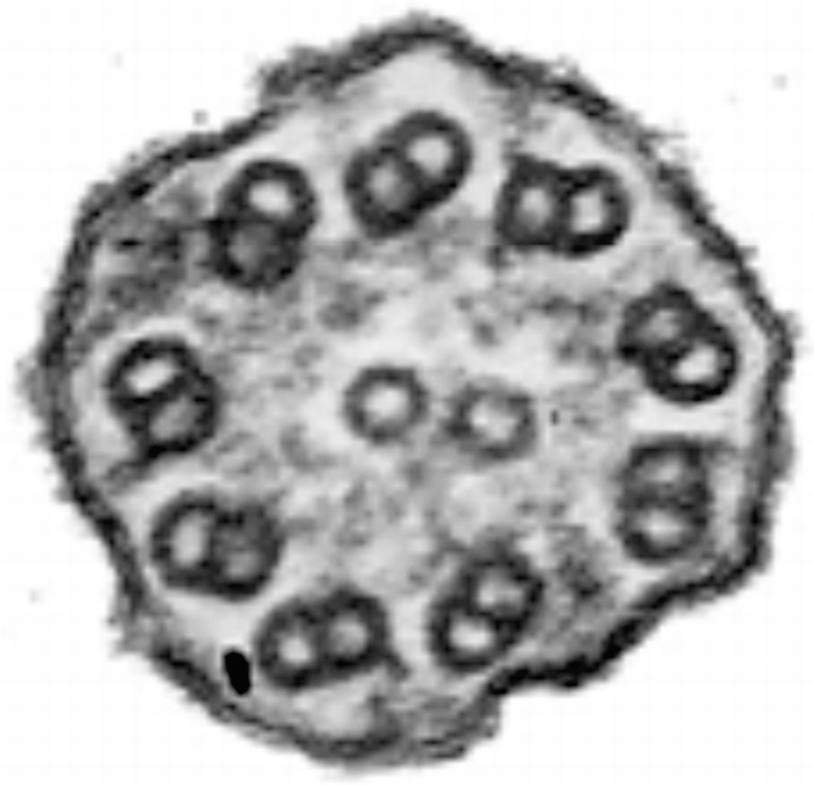
Ciliary dyskinesia. Cross section of cilium showing complete absence of dynein external and internal arms in peripheral microtubules. Uranyl acetate and lead citrate. ×120,000
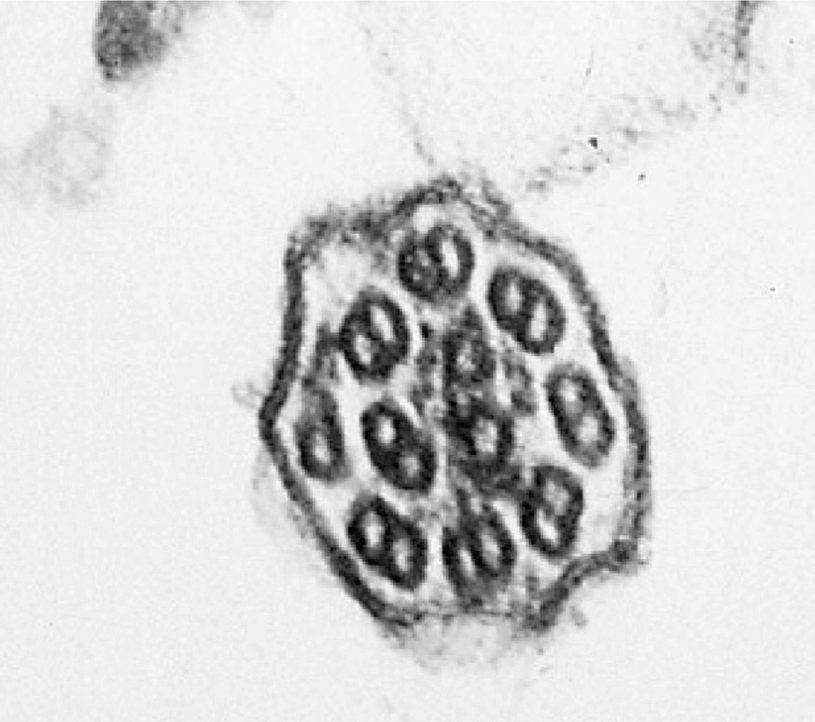
Ciliary dyskinesia
The most frequent ultrastructural findings, as published in several studies, are the absence of both dynein arms (range, 24–57%), absence of only the external dynein arm (range, 2.9–43%), absence of only the internal dynein arm (range, 29–37%), and anomalies of the central microtubules (4%).
Ultrastructural diagnosis requires trained and experienced specialists, as well as the strict application of morphological and diagnostic criteria. Diagnosis is more reliable when samples have been optimally obtained and processed. Up to 40% of the samples can be labeled as useless, as they do not contain ciliary cells in adequate numbers and lack preservation quality.
Clinical Condition
Clinical characteristics in PCD depend on the age of the patient.
Situs inversus or heterotaxia in antenatal echography
Mild brain ventriculomegaly in antenatal echography
Respiratory distress in term newborn, without risk factors for transitory tachypnea or neonatal pneumonia
Permanent nasal congestion since the first day of life
Situs inversus or dextrocardia
Complex congenital heart disease related to laterality disorders (heterotaxia)
Biliary atresia
Hydrocephalus
Persistent wet cough
Atypical wheezing that does not respond to steroids therapy
Recurrent pneumonia
Bronchiectasis of unclear etiology
Frequent rhinosinusitis
Otitis media with chronic effusion
Persistent otorrhea after the insertion of ventilation tubes
Situs inversus associated to sinusitis and bronchiectasis
Severe gastroesophageal reflux
The same manifestations as present during school-age period
Greater percentage of ectopic pregnancies and reduced fertility in women
Greater percentage of infertility in men
Chronic mucopurulent bronchorrhea
Digital clubbing
Nasal polyposis/halitosis
Function tests show mixed or obstructive pattern
The clinical manifestations most commonly published in the different series are recurrent sinusitis (77%), persistent rhinitis (76%), neonatal respiratory distress syndrome or tachypnea (20–67%), recurrent otitis media (57%), recurrent pneumonia (46–56%), difficult-to-control asthma (26%), bronchiectasis (8.6–24%), and situs inversus (18–69%). The average age at diagnosis is 4 years, but with a high clinical suspicion it can be diagnosed earlier, even within the first month of life.
Diagnosis
The diagnosis of PCD is based on a combination of clinical evaluation and analysis of the ciliary ultrastructure and function, and it can be difficult to determine this diagnosis in some patients. The clinical phenotype is wide and overlaps with other chronic diseases of the airway.
The patient must be thoroughly studied to rule out other pathologies (allergies and immunological studies, X-rays, gastroesophageal reflux, sweat test, etc.) that may be responsible for the clinical condition.
The screening and diagnosis methods for ciliary dyskinesia are detailed next.
Screening Methods
- a.
Saccharine test: A piece of saccharine placed in the lower part of the nostril will migrate toward the pharynx by the ciliary movement. The patient should be able to perceive the taste in the mouth before 30 min. The precision of this test is poor; it depends on several variables, and it has no value in children under 10 years old. The result is considered to be openly abnormal if the patient needs 60 min or more in perceiving the saccharine taste.
- b.
Technetium-99 m-labeled colloid albumin test : 50 mCi technetium-99 dissolved in 2.5 μl saline solution is placed in the lower part of the nostril, 1 cm deep. The migration of the tracer is measured with a gamma camera. The result is abnormal if there is no tracer movement within 10 min. It can be used in children under 10 years old. Sensitivity is 100%, but it has a moderate specificity. It has a low positive predictive value but perfect negative predictive value.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


