Chapter 55 Chronic Venous Insufficiency
An estimated 25 to 40 million Americans have varicose veins, and more than 2 million have chronic venous insufficiency, including venous edema, skin changes, or venous ulcers.1 The cost of venous ulcer care is greater than $3 billion in the United States; in the United Kingdom, estimates are greater than $1 billion annually.2,3 Patients with advanced venous disease have decreased quality of life and occasionally, severe disease that is limb threatening.4,5
Definition
Chronic venous disease (CVD) includes a series of clinical conditions of varying severity, from varicose veins at one end of the spectrum to venous ulcers at the other. Chronic venous insufficiency (CVI) is the diagnosis given to patients with venous dysfunction causing edema, skin changes, or ulcerations (CEAP class 3-6; Box 55-1). The CEAP classification is used to define, categorize, and grade the severity of all chronic venous disorders.6 This classification provides insight into the clinical presentation (C), etiology (E), anatomy (A), and pathophysiology (P) of the underlying venous disorder.
![]() Box 55-1 The CEAP Classification (Basic)
Box 55-1 The CEAP Classification (Basic)
2. Etiological classification:
4. Pathophysiological classification:
Adapted from Eklof B, Rutherford RB, Bergan JJ, et al: Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg 40:1248–1252, 2004; used with permission.
Clinical Presentation
Varicose veins (CEAP class C2) are dilated superficial veins (>3 mm in diameter in the upright position)7 (see Chapter 54). Chronic venous insufficiency includes patients with more advanced disease, with or without varicose veins. These patients have edema, skin changes like dermatosclerosis, pigmentation, corona phlebectatica or eczema, or healed or active venous ulcer8,9 (see Box 55-1).
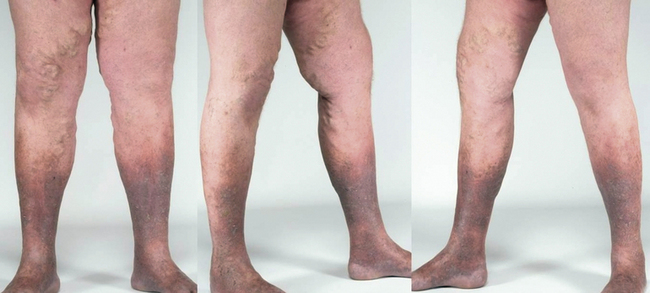
Increased ambulatory venous hypertension leads to a series of changes in subcutaneous tissue and skin, mostly in the “gaiter” area of the leg, at or above the ankle (Fig. 55-1). These are caused by activation of the endothelial cells (ECs), extravasation of macromolecules and red blood cells, diapedesis of leukocytes, tissue edema, and chronic inflammatory changes.7 These changes are all consequences of either venous valvular incompetence or venous obstruction, or a combination of both.
Etiology
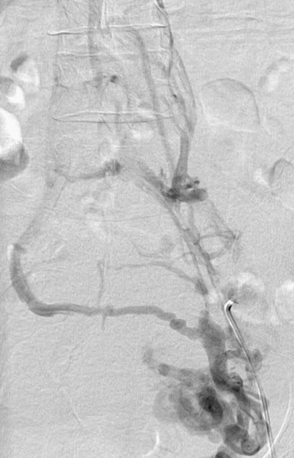
Most cases of venous insufficiency have underlying primary etiology. The most frequent cause is likely intrinsic morphological or biochemical abnormality in the vein wall. The origin of venous reflux in patients with primary varicose veins can be local or multifocal structural weakness of the vein wall, and this can occur together or independently of proximal saphenous vein valvular incompetence.10 Chronic venous insufficiency can also develop as a result of secondary causes such as previous deep venous thrombosis (DVT), deep venous obstruction, superficial thrombophlebitis, or arteriovenous fistula (AVF). May-Thurner’s syndrome (occlusion or stenosis of the left iliac vein due to compression by the overriding right common iliac artery [CIA]) is a cause of CVI that is likely much more frequent than previously thought (Fig. 55-2). Varicose veins may also be congenital and present as venous malformation and eventually lead to CVI. Primary venous insufficiency accounts for approximately 70% of advanced CVI (class 4-6), with the remainder occurring in legs following DVT.11,12
Anatomy
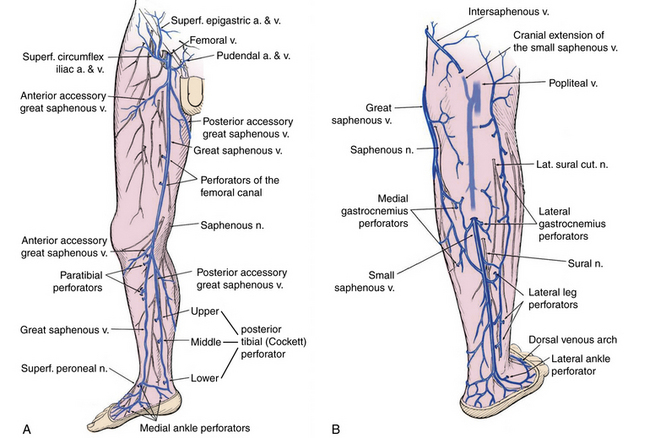
Significant changes have been made to venous terminology over the last decade, and these have been uniformly adopted and promoted by international vascular societies.13,14Superficial veins of the lower limbs are those located between the deep fascia, covering the muscles of the limb, and the skin. The main superficial veins are the great saphenous vein (GSV) and the small saphenous vein (SSV; Fig. 55-3). All previous names used to describe these vessels (greater, long, and lesser) should be abandoned. The GSV originates from the medial superficial veins of the dorsum of the foot and ascends in front of the medial malleolus along the medial border of the tibia, behind the saphenous nerve. There are posterior and anterior accessory saphenous veins in the calf and the thigh. The saphenofemoral junction (SFJ) is the confluence of superficial inguinal veins comprising the GSV and superficial circumflex iliac, superficial epigastric, and external pudendal veins. The GSV in the thigh lies in the saphenous subcompartment of the superficial compartment between the saphenous fascia and deep fascia. The SSV is the primary superficial vein of the posterior aspect of the lower leg and originates posterior to the lateral malleolus and courses posterolaterally in the lower leg to drain into the popliteal vein, behind the knee. The intersaphenous vein (vein of Giacomini) connects the SSV in the posterior thigh with the GSV.15
Deep veins follow the arterial circulation in the limb and pelvis and are usually paired in the lower leg, accompanying tibial arteries by the same name. The popliteal or the femoral vein can also be paired. The femoral vein runs parallel to the superficial femoral artery (SFA) and replaces the term superficial femoral vein to avoid confusion regarding its importance as a deep vein.14 The pelvic veins include the external, internal, and common iliac veins (CIVs), which drain into the inferior vena cava (IVC). Large gonadal veins drain into the IVC on the right and left renal vein on the left.
Perforator veins traverse the deep muscular fascia and communicate between the superficial and deep venous system at multiple levels in the lower extremity (see Fig. 55-3). These primarily function to drain the superficial system into the deep venous system. The most important leg perforating veins are the medial calf perforators.16 The posterior tibial perforating veins (“Cockett perforators” in the old nomenclature) connect the posterior accessory GSV with the posterior tibial veins and form the lower, middle, and upper groups. They are located just behind the medial malleolus (lower), at 7 to 9 cm (middle) and 10 to 12 cm (upper) from the lower edge of the malleolus. The distance between these perforators and the medial edge of the tibia is 2 to 4 cm.16 Paratibial perforators connect the main GSV trunk with the posterior tibial veins. In the distal thigh, perforators of the femoral canal connect, usually directly, the GSV to the femoral vein.
Bicuspid venous valves are present in all superficial and deep lower-extremity veins and are important in assisting normal unidirectional venous flow. The GSV usually has at least six valves (maximum 14-25),17 with a constant valve present within 2 to 3 cm of the SFJ in 85% of cases (preterminal valve).18 The SSV has a median of 7 to 10 valves (range, 4-13).17 There are valves in the deep veins of the lower limb, but the common femoral or external iliac vein has only one valve in about 63% of cases.17 In 37%, there is no valve in the common iliac vein. The internal iliac vein has a valve in 10%; its tributaries have valves in 9%.19
Although isolated severe incompetence of the superficial system may lead to development of ulcers with high ambulatory pressures, most venous ulcers have underlying multisystem (superficial, deep, perforator) incompetence involving at least two of the three venous systems.20,21 Of 239 patients with venous ulcers evaluated with duplex scanning in three different studies, 144 (60.3%) had incompetent perforating veins, and 141 (59%) had deep vein incompetence or obstruction.22–24
Pathophysiology
Most patients with CVI have primary or secondary venous valvular incompetence. Venous outflow obstruction can be the result of primary venous disease (e.g., May-Thurner’s syndrome),25 or it can be the result of a previous DVT. Occasionally, congenital anomalies like deep vein agenesis or hypoplasia result in venous outflow obstruction.
Recent evidence suggests that venous obstruction could be more important than valvular incompetence. Among 504 patients who underwent iliac vein stenting for venous obstruction, symptoms resolved after stenting, despite associated valvular incompetence.26
Diagnostic Evaluation
A thorough history and physical examination should be complemented by duplex scan of the superficial and deep veins to evaluate obstruction and valvular incompetence, as recommended by the recent guidelines of the Society for Vascular Surgery and the American Venous Forum (recommendation grade 1A).27 Subsequent diagnostic testing is carried out based on clinical presentation and examination findings.
History
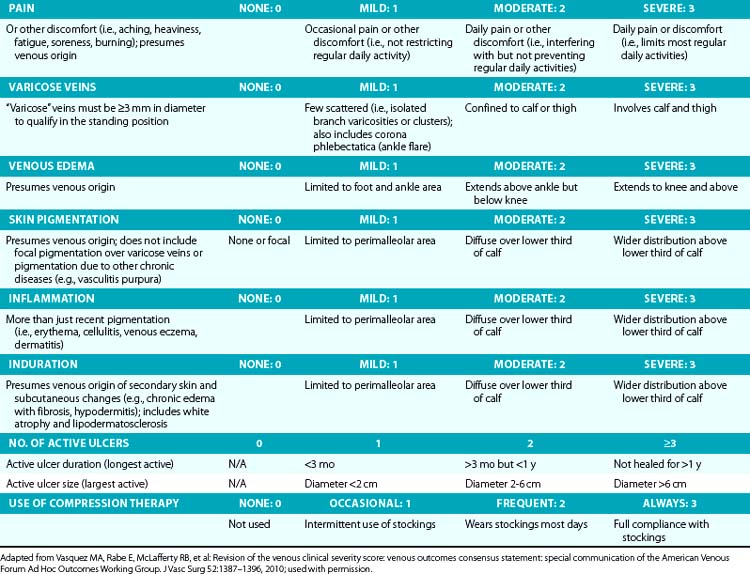
Symptoms related to CVI can include tingling, aching, burning, pain, muscle cramps, swelling, sensation of throbbing or heaviness, itching skin, restless legs, leg tiredness, fatigue, or ulceration in extreme cases.28 These symptoms suggest CVI, particularly if patients notice exacerbation by heat or dependency during the course of the day and relief by resting or leg elevation or use of compression therapy.29 Pain during and after exercise that is relieved with rest and leg elevation (venous claudication) can also be caused by venous outflow obstruction from previous DVT (postthrombotic syndrome) or obstruction of CIVs (May-Thurner’s syndrome).28 Diffuse pain is more frequently associated with axial venous reflux, whereas poor venous circulation in bulging varicose veins usually causes local pain. The Guideline Committee recommends using the revised Venous Clinical Severity Score (VCSS; Table 55-1)30 to grade and document the presenting symptoms of patients with CVD.27
A detailed medical history may establish the diagnosis of primary, secondary, or congenital venous problems. Adequate history should address previous DVT or thrombophlebitis, personal or family history of thrombophilia, medication history (particularly oral contraceptive pills), smoking, obstetric history, and a family history of venous disorders (most patients with varicose veins would be able to relate their parents’ or grandparents’ disease). Premenopausal women with varicose veins should also be questioned for symptoms of pelvic congestion syndrome (pelvic pain, aching, or heaviness; dyspareunia). Advanced age is the most important risk factor for varicose veins and CVI. A positive family history and obesity are risk factors for CVI.31
Physical Examination
Examination should always be performed with the patient in the standing position in a warm room with good light, and should establish the size, location, and distribution of varicose veins and also focus on other signs of venous disease such as edema (partially pitting or nonpitting), skin changes (induration, pigmentation, lipodermatosclerosis, atrophie blanche, eczema, dermatitis, skin discoloration, increased skin temperature), and ulceration (healed or active). Inspection and palpation are essential parts of the examination, and auscultation (for bruits) is particularly helpful in those with vascular malformation and arteriovenous fistula.32 Varicose dilations or venous aneurysms, palpable cord in the vein, tenderness, thrill, bruit, or pulsatility should be recorded. Ankle mobility should also be examined because patients with advanced venous disease frequently have decreased mobility in the ankle joints. Sensory and motor functions of the limb and foot are assessed to help differentiate from diabetic neuropathy or any underlying neurological problem. An abdominal mass or lymphadenopathy can provide a clue to venous compression and outflow obstruction.
Corona phlebectatica (ankle flare or malleolar flare) is a fan-shaped pattern of small intradermal veins located around the ankle or the dorsum of the foot.27 This is considered to be an early sign of advanced venous disease. Inspection of the abdominal wall and perineal and inguinal region should be routinely performed. Perineal, vulvar, or groin varicosities can be seen in iliac vein obstruction or internal iliac vein or gonadal vein incompetence causing pelvic congestion syndrome. Scrotal varicosity may be a sign of gonadal vein incompetence, left renal vein compression between the superior mesenteric artery (SMA) and aorta (nutcracker syndrome), or occasionally even IVC lesions or renal carcinoma.
Classic tourniquet tests for saphenous or perforator incompetence or deep venous occlusion (Trendelenburg test, Ochsner-Mahorner test, Perthes test) are rarely used today. They are mostly of historical interest and should be used in rare instances when duplex scanning or Doppler studies are unavailable.32 Distal palpation and proximal percussion of the saphenous vein, however, are useful tests to suggest valvular incompetence.
Skin lesions other than those already described (e.g., capillary malformations, tumors, onychomycosis, excoriations) should also be noted. An aneurysmal saphenous vein can be misdiagnosed as a femoral hernia or vice versa. Presence of a longer limb, lateral varicosity noted soon after birth, and associated capillary malformations are tipoffs for congenital venous malformation (Klippel-Trénaunay’s syndrome).33 Edema of the dorsum of the foot, squaring of the toes, thick skin, and nonpitting edema are signs of chronic lymphedema. A complete pulse examination should be performed to exclude underlying peripheral arterial disease. The physical examination can be complemented by a handheld Doppler examination, although the latter does not replace evaluation of the venous circulation with color duplex scanning.
Duplex Scanning
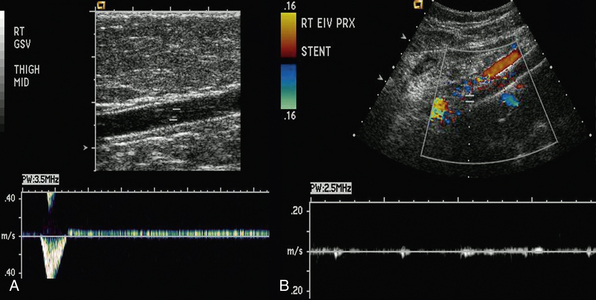
Duplex scanning is safe, noninvasive, cost-effective, and reliable and is recommended as the first diagnostic test for all patients with suspected CVD27,34,35 (also see Chapter 12). B-mode imaging permits accurate placement of the pulsed Doppler sample volume, and color flow evaluation makes it easier to establish obstruction, turbulence, and direction of venous and arterial flow,36 increasing diagnostic accuracy in comparison to continuous wave Doppler ultrasonography in the assessment of venous insufficiency.37 Duplex scanning is excellent for evaluation of both infrainguinal venous obstruction and valvular incompetence38 (Fig. 55-4).
The appropriate technique of venous duplex scanning has been described in detail by several authors.39 An 8.4- to 9-MHz linear array pulsed-wave Doppler transducer is used most frequently for the deeper veins, with the higher-frequency probe (up to 18 MHz) for detailed assessment of the superficial veins. Evaluation of reflux should be performed with the patient in the upright position, with the leg rotated outward, heel on the ground, and weight taken on the opposite limb.35 The supine position gives both false-positive and false-negative results of reflux.40 All deep veins of the leg are evaluated, followed by evaluation of the superficial veins, including the GSV, SSV, accessory saphenous veins, and perforating veins for a complete examination.
The four essential components of a complete duplex scanning examination for CVD are visibility, compressibility, venous flow, and augmentation. Asymmetry in flow velocity, lack of respiratory variations in venous flow, and waveform patterns at rest and during flow augmentation in the common femoral veins indicate proximal obstruction. Reflux can be elicited in two ways: (1) increased intraabdominal pressure using a Valsalva maneuver for the common femoral vein or SFJ or (2) manual/cuff compression and release of the limb distal to the point of examination. The first is more appropriate for evaluation of reflux in the common femoral vein and at the SFJ, whereas compression and release is the preferred technique more distally on the limb.40 The guidelines recommend the cutoff value for abnormally reversed venous flow (reflux) as 1.0 second in the deep femoropopliteal veins and 500 milliseconds in the superficial and perforator veins.27
Perforating veins are evaluated in patients with advanced disease, usually CEAP class C5-C6, or in those with recurrent varicose veins after previous interventions. The diameter of a clinically relevant “pathological perforator” (e.g., beneath healed or open venous ulcer) can predict valve incompetence. The SVS/AVF Guideline Committee definition of “pathological” perforating veins includes those with outward flow of more than 500 milliseconds, with a diameter of greater than 3.5 mm, located beneath a healed or open venous ulcer (CEAP class C5-C6).27
Plethysmography
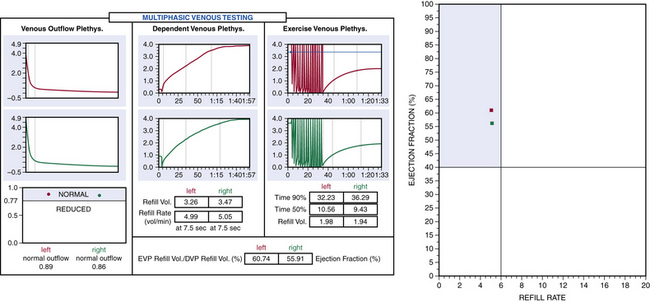
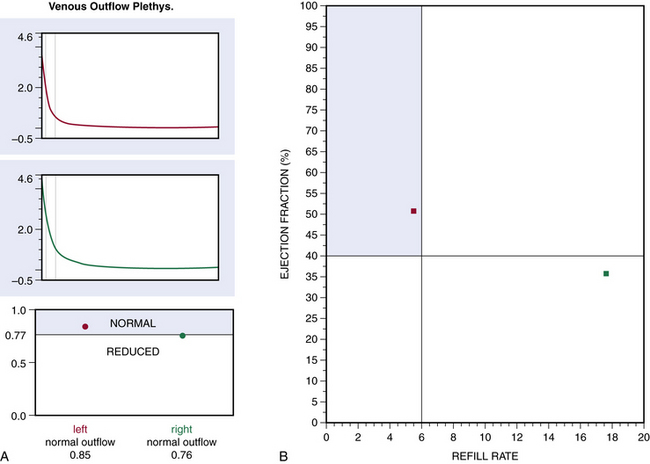
Air or strain-gauge venous plethysmography can provide noninvasive evaluation of calf muscle pump function, global venous reflux, and venous outflow obstruction41–44 (Figs. 55-5 and 55-6). Strain-gauge plethysmography is usually performed with a modified Struckmann protocol, validated by comparison with simultaneously recorded ambulatory venous pressure measurements.45 Strain-gauge or air plethysmography consists of exercise venous plethysmography, measurement of passive refill and drainage, and outflow plethysmography. Plethysmography quantifies venous reflux and obstruction and has been used to monitor venous functional changes and assess physiological outcome of surgical treatments.46,47 Venous plethysmography provides information on venous function in patients with CVI and is a complementary examination to duplex scanning. These studies are especially helpful in patients with suspected outflow obstruction but normal duplex findings, or those suspected of having venous disease due to calf muscle pump dysfunction, but no reflux or obstruction was noted on duplex scanning. Air plethysmography remains one of the few noninvasive techniques that can quantify reflux; other parameters have been reported to be variably useful.44,47 According to the new guidelines, use of air plethysmography is encouraged as best practice in evaluation of patients with CVI (C3-6).27
Intravascular Ultrasound
Recent assessment of patients with iliofemoral venous occlusion suggested that intravascular ultrasound (IVUS) should be used for evaluation of all patients with suspected or confirmed iliac vein obstruction. Intravascular ultrasound can be used in veins with obstruction without occlusion to assess venous wall morphology and mural thickness and identify trabeculations and recanalization, frozen valves, and external compression. Some of these lesions, as emphasized by Raju and Neglen, are not seen with conventional planar venography and provide measurements in assessing the degree of stenosis.48 In addition, in patients who have had an endovenous intervention, IVUS confirms position of the stent in the venous segment and resolution of the stenosis.48
Contrast Venography and Hemodynamic Studies
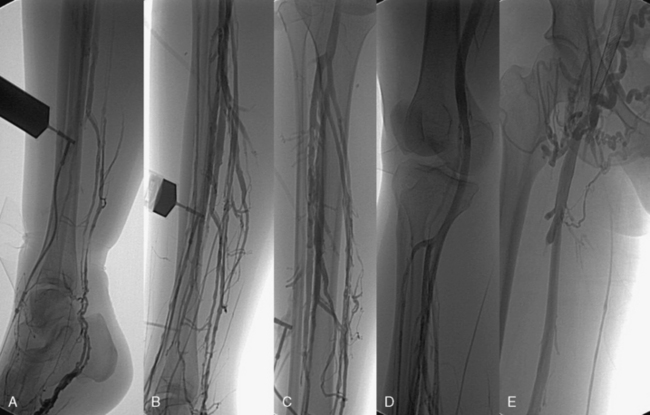
Ascending or descending (or both) contrast venography for CVI is performed selectively in patients with deep venous obstruction, postthrombotic syndrome, thrombotic or nonthrombotic pelvic vein obstruction (May-Thurner’s syndrome), pelvic congestion syndrome, nutcracker syndrome, vascular malformations, venous trauma, tumors, and if endovenous or open surgical treatment is planned. Descending venography is used to study venous valve incompetence. The Valsalva maneuver is used to grade the severity of reflux (grade 1-4: 1 = to upper thigh, 2 = to distal thigh, 3 = popliteal reflux, 4 = reflux to tibials and perforators).49,50 Since open valve repair is rarely performed today, this test has been less frequently used in recent years. Ascending venography is performed in a standing position to evaluate patency of the superficial and/or deep venous system (Fig. 55-7). It can be used together with direct venous pressure measurements to evaluate patients with varicose veins and associated iliac vein obstruction (May-Thurner’s syndrome). Contrast venography is routinely used in CVD to perform endovenous procedures such as angioplasty or venous stenting or open venous reconstructions. A pressure gradient across iliofemoral obstruction at rest in the supine patient (3 mmHg) is indicative of functional venous obstruction. Arm/foot venous pressure measurements and ambulatory venous pressure (AVP) measurement in a dorsal foot vein are additional tests that can be performed. Detailed descriptions and techniques for these tests are provided in the consensus statement.2
Computed Tomography and Magnetic Resonance Venography
Early venous disease (C1-2) rarely requires advanced imaging studies other than duplex ultrasonography. Computed tomography (CT) and magnetic resonance imaging (MRI) have progressed tremendously in the last decade and provide excellent three-dimensional (3D) imaging of the venous system. Both modalities are suitable to identify pelvic or iliac venous obstruction in patients with lower-limb varicosity when proximal obstruction or iliac vein compression (May-Thurner’s syndrome) is suspected.48 They are also suitable to establish left renal vein compression (nutcracker syndrome),51 gonadal vein incompetence, and pelvic venous congestion syndrome. Gadolinium-enhanced MRI is especially useful in evaluating patients with vascular malformations, including those with congenital varicose veins.52
Laboratory Evaluation
Based on their history, selected patients with recurrent DVT, thrombosis at a young age, or thrombosis in an unusual site should undergo screening for thrombophilia.27 Laboratory examination is also needed in patients with long-standing recalcitrant venous ulcers, since a small percentage of these patients could have an underlying secondary etiology, including neoplasia, chronic inflammation, and other disorders.53 Patients who undergo general anesthesia for treatment of CVI should undergo appropriate testing to assess suitability for such procedures.
Severity of Venous Disease
The diagnostic evaluation should provide adequate information to quantify and classify the severity of venous disease, using CEAP clinical class and VCSS.6,30 The revised VCSS documents are used to establish disease severity at the first examination and will quantify improvement or deterioration during follow-up. In the basic CEAP classification, only the highest score is used to denote clinical class, and only the main anatomical groups (superficial, perforating, deep) are noted.
The revised format of the classification6 includes two elements in addition to the CEAP findings, the date and diagnostic level of the evaluation:
l. Level 1: history, physical, Doppler examination (handheld).
l. Level 2: noninvasive—duplex scan, plethysmography.
l. Level 3: invasive—venography, venous pressure, IVUS, CT venography, MR venography.
The main purpose of using the CEAP classification in patients with varicose veins is to distinguish primary venous disease causing simple varicose veins from secondary postthrombotic venous insufficiency.7 Evaluation and treatment of the two conditions are distinctly different. Complete CEAP classification and quality-of-life (QOL) evaluation is recommended before and after treatment to help to assess the patient’s perception of the burden of the disease for research purposes. A general QOL instrument such as the SF-36 and one of the disease-specific QOL instruments (e.g., VEINES, CIVIQ 2, Aberdeen, Charing cross) should be used.4,54–56
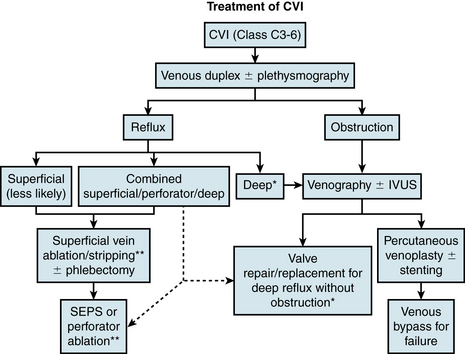
Treatment of Chronic Venous Insufficiency
Treatment of CVI requires a multimodality approach and has roles for both medical and interventional therapy. A concise flowchart for surgical/interventional treatment decision making is presented in Figure 55-8.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree