C0 – No signs of visible or palpable venous disease
C1 – Telangectasias or reticular veins
C2 – Varicose veins
C3 – Edema
C4a – Pigmentation, eczema
C4b – Lipodermatosclerosis or atrophie blanche
C5 – Healed venous ulcer
C6 – Open venous ulcer
S – Symptomatic, ache, pain, tightness, skin irritation, heaviness, muscle cramps
A – Asymptomatic
Diagnosis
The diagnosis of CVI derives from the history and physical exam along with adjunctive information from noninvasive testing. The history should characterize the presence, duration, and severity of symptoms including edema, pain, fluid drainage, and skin breakdown. Patients should be questioned about previous thrombotic episodes and evaluated for the presence of thrombotic risk factors such as family history, personal traits, and medication use. Documenting previous treatments for CVI that have succeeded or failed can help in the making of decisions regarding future therapy.
Physical examination begins with visual inspection and palpation of the lower extremity skin. Dilated, tortuous varicose veins may be obvious at first glance, or they have a more subtle appearance as localized, palpable skin bulges. The upright posture will maximally distend the veins making them easier to detect and characterize. Cutaneous changes associated with CVI range from hyperpigmentation to fibrosis to frank ulceration (Fig. 11.1). Sites of healed venous ulceration referred to as “atrophie blanche” appear as a localized area of white scarring with an absence of capillaries. Edema usually gives way to palpation and is described as “pitting edema.” Patients with long-standing CVI and underlying fibrosis often develop brawny edema that does not indent with palpation.
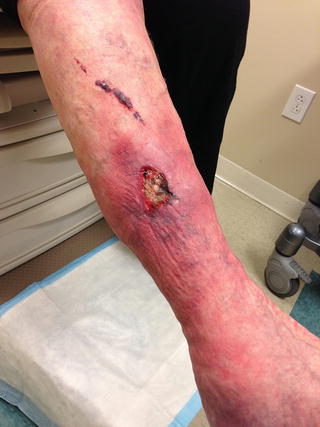

Fig. 11.1
Chronic venous ulcer of the lower extremity
Simple bedside maneuvers can help detect the presence and nature of venous reflux. In the Trendelenburg or tourniquet test, the veins of leg are first emptied by elevating the lower extremity with the patient supine [13]. The patient then assumes an upright position with a tourniquet or manual pressure applied to various levels. In the presence of superficial venous reflux, the varicose veins distal to the tourniquet will remain collapsed until the tourniquet is released. With deep venous reflux, varicose veins immediately reappear upon standing despite the presence of a tourniquet or manual compression. More detailed information regarding venous reflux can now be gained from duplex ultrasound examination making bedside maneuvers a less important component of the physical exam.
Before making a definitive diagnosis of CVI, other conditions should be considered in the differential. The most serious cause of limb edema is acute DVT which can be detected with a duplex ultrasound exam. Systemic causes of lower extremity edema include congestive heart failure, renal insufficiency, liver disease, endocrine disorders, and medication side effects. Localized conditions such as a ruptured popliteal cyst, hematoma, exertional compartment syndrome, gastrocnemius muscle tear, and lymphedema can also cause lower extremity edema. A carefully performed history and physical exam and appropriately selected noninvasive tests can usually clarify the diagnosis.
Noninvasive Testing
Lower extremity venous duplex ultrasound exams first evaluate for acute thrombosis by checking the patency of the deep and superficial venous systems. Sonographic evidence of a previous thrombotic event can take the form of intraluminal echoes, thickened vein walls, and prominent venous collaterals. These chronic venous changes would support an obstructive etiology for CVI. Diagnosing venous reflux involves measuring the duration of reversed blood flow at various locations. The standard venous reflux exam involves inflation and rapid deflation of cuffs on the lower extremity with the patient standing. The duration of reversed flow after cuff deflation corresponds to the valve closure time [14]. In the deep venous system, abnormal reflux is defined as reversed flow longer than 1.0 s, while the threshold for reflux is 0.5 s in the superficial venous system. Reflux can also be elicited using the Valsalva maneuver with the patient supine in 30° reversed Trendelenburg position. Patients with persistent stasis dermatitis or recalcitrant venous ulcers, especially those who have already been treated for axial (great and/or small saphenous vein) reflux, should be evaluated for perforator reflux. Reversed flow going from deep to superficial veins lasting longer than 0.3 s indicates reflux in the perforating venous system.
Although duplex ultrasonography can detect venous reflux, the results of the exam do not correlate with the clinical manifestations or severity of CVI [15]. Other less widely used noninvasive exams provide a more complete assessment of the venous hemodynamics in the lower extremity. Air plethysmography (APG) uses pressure cuffs and physical maneuvers to measure the variables that contribute to CVI: obstruction, reflux, and muscle pump dysfunction. During an APG exam, changes in the volume of air displaced in air-filled cuffs quantifies the venous outflow fraction, refilling index, and ejection fraction. A low venous outflow fraction correlates with obstruction; a high refilling index detects reflux; and a poor ejection fraction indicates muscle pump dysfunction [16]. APG can determine how much each variable contributes to CVI making APG a valuable tool in planning interventions and assessing the response to treatment. Photoplethysmography is a more convenient but less quantitative method for detecting venous reflux and assessing overall venous function.
Invasive Testing
Invasive testing rarely plays a role in the diagnosis of CVI. Contrast venography involves a lower extremity venipuncture and the injection of radiopaque contrast. Descending venography using femoral vein access usually employs a tilt table to evaluate for reflux in the femoral and great saphenous veins. Ascending venography injects contrast through a vein on the dorsum of the foot to assess venous anatomy and patency. Once considered the diagnostic standard, venography has been almost completely replaced by noninvasive exams. Venograms are now most commonly performed in conjunction with venous thrombolytic procedures. In the rare patient being considered for venous reconstruction, venography may be helpful in planning surgery or clarifying inconclusive ultrasound images.
Lower extremity venous cannulation can also be used to measure ambulatory venous pressure (AVP). After placing a catheter in a vein on the dorsum of the foot, the venous pressure is measured in various positions and before and after walking and toe raises. These exercises yield several physiologic parameters including mean ambulatory pressure and refill time. Previous studies suggest that AVP and the results generated correlate with CVI severity, risk of ulceration, and response to treatment [17]. Other studies have questioned the value of AVP especially in light of noninvasive exams which have reasonable accuracy in evaluating overall venous competence [18]. The performance of this study is not commonplace.
Treatment
Treatment for CVI ranges from conservative measures to invasive procedures depending on disease severity. All patients with CVI should adopt behavioral modifications designed to minimize lower extremity edema including leg elevation, avoidance of prolonged standing, and weight loss (if indicated) to reduce intra-abdominal pressure. Conservative therapy consisting of external compression usually suffices for patients with mild venous disease. Patients with more severe manifestations of CVI (CEAP class 4 to 6) may warrant referral to a vein specialist to consider interventional therapy. Even patients with CEAP class 3 and extensive edema may benefit from more aggressive treatment to reduce the risk of recurrent skin breakdown and nonhealing venous ulcers.
Noninterventional Therapy
Compression stockings exert an external force on the leg to oppose the hydrostatic pressure caused by venous hypertension. Graded compression stockings exert the most force at the ankle and decrease in pressure as they go up the leg. By keeping blood from pooling in the lower leg, compression stockings usually improve symptoms related to venous congestion such as edema, leg fatigue, and aching discomfort. In addition to symptomatic relief, compression stockings may also have physiologic benefit by improving muscle pump function and reducing venous reflux.
Prescriptions for compression stockings should specify the tension and length. Tension varies with clinical severity starting with 20–30 mmHg for patients with mild edema, 30–40 mmHg for CEAP class 4–6, and 50 mmHg for patients with recurrent venous ulcers. Knee-high stockings are the easiest to use and usually provide symptomatic relief. Although thigh-high and waist-high stockings may be indicated for patients with extensive edema, they are cumbersome and usually generate patient complaints and noncompliance. Measuring leg diameter at the ankle, calf, and thigh improves the fit of compression stockings and can be performed in the office or at the medical supply facility.
Patients should be instructed to apply the stockings as soon as they get up in the morning when the lower extremities have the least edema. The stockings should be worn throughout the day and taken off in the evening before bed. Education about stockings should emphasize the importance of daily use as patient compliance has a significant impact on treatment success. With regular use, compression stockings lose their elasticity in 6–9 months and should be replaced accordingly.
Used regularly, compression stockings can relieve the symptoms of CVI even to the point of healing venous ulcers. Unfortunately, noncompliance often prevents patients from achieving the full benefit of compression therapy. Barriers that keep patients from regularly wearing compression stockings include lack of physical strength, arthritis, inconvenience, and ulceration or drainage requiring frequent dressing changes. Pain can also be a factor in noncompliance as some patients have too much lower extremity discomfort to tolerate any external compression. Other medical devices have been designed to address lower extremity edema. Although twice daily use of external compression pumps can be effective, compression stockings are often necessary to prevent edema from returning between treatment sessions.
Dry skin associated with CVI requires moisturizers, while topical steroids may be necessary to treat stasis dermatitis. Left unchecked, compromised skin can break down to form a venous ulcer, the most severe and difficult to heal manifestation of CVI. Treatment of the ulcer begins by controlling edema and infection. Nonviable tissue must be debrided to healthy tissue with either pharmacologic agents or sharp debridement. The formation of granulation tissue and neovascularization in the wound bed allows for epithelialization and healing. Silver-impregnated dressings can help manage wounds affected by infection or bacterial overgrowth.
Although wound care promotes healing, venous ulcers do not usually resolve without compression to control edema. The Unna boot has been used for over a century to apply external compression to patients with lower extremity wounds. Medicated dressings form the first layer of an Unna boot followed by a noncompliant wrap to exert compression. Unna boots have the advantage of being able to stay in place for up to a week at a time; however, the wraps tend to loosen and exert less pressure as time passes. Four-layer wraps maintain their strength longer and have been shown to promote ulcer healing [19]. Blair et al. found that 82 % of patients using four-layer wraps had healed their ulcers within 20 weeks.
Used in conjunction with external compression, bioengineered skin substitutes can accelerate ulcer healing rates compared to control. Split-thickness skin grafting can also be considered in selected patients.
Medical therapy offers a potentially promising treatment for CVI. Coumarins, flavonoids, saponosides, and other plant extracts have venoactive properties that may improve venous tone and decrease capillary permeability. Although these medications are used in Europe, they have not been approved for use in the United States. Pentoxifyllene which is normally used in the treatment of claudication may improve venous ulcer healing rates. The magnitude of this effect appears to be small and pentoxifyllene does not currently have a well-defined role in the treatment of CVI [20]. Exercise programs also have an unclear role in the management of CVI. Directed rehabilitation programs improve muscle pump function, but they do not decrease the amount of venous reflux [21].
Interventional Therapy
Healing for venous ulcers often proves to be fleeting. Severe ulcers can become completely refractory to conservative measures, and recurrence after healing is as high as 80 % in the first year [22]. Patients who have nonhealing ulcers or persistent disability and ulcer recurrence despite maximal noninvasive therapy should be considered for surgical or endovascular interventions. Interventional techniques attempt to correct the underlying pathophysiology of CVI by minimizing venous reflux and relieving venous obstruction.
Superficial venous reflux increases lower extremity edema, promotes varicose vein formation, and exacerbates deep venous reflux. Eliminating superficial reflux improves venous hemodynamics which provides symptomatic relief and may assist in ulcer healing. In a randomized study of patients with venous ulcers, surgery to eliminate superficial venous reflux reduced the rate of ulcer recurrence by more than half compared to compression therapy alone. Interventions for superficial venous reflux including saphenous vein stripping, endovenous ablation, and sclerotherapy can be applied to all CEAP clinical classes 2–6. Chapter 12 has a full description of these techniques.
Unlike superficial venous reflux, reflux in the deep veins cannot be eliminated by surgical stripping or endovenous ablation procedures. A small number of vascular specialists perform surgery to restore deep vein valvular competency in highly selected patients with recurrent venous ulcers and severe reflux. Surgical techniques for valvular reconstruction include external valvuloplasty, brachial/axillary valve transfer, and vein transpositions [23, 24].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


