Chapter 59
Chronic Venous Insufficiency
Treatment of Perforator Vein Incompetence
Fedor Lurie, Alessandra Puggioni
The perforating veins (PVs) were not described until the drawings of Justus Christian Von Loder were published in 1794.1 From that time for more than a century, their role in venous disease remained unappreciated despite the illuminating work of M. Verneuil and subsequent analysis by John Gay.2 In 1917, John Homans suggested that incompetent perforating veins (IPVs) play a key role in the genesis of venous ulcers. Subsequently, Beecher and Warren formulated the concept of transmission of ambulatory pressure through IPVs into the superficial system, which was supported by the investigations of Cockett and Dodd and provided a physiologic basis for the surgical interruption of IPVs. In 1938, Linton described a surgical approach to IPVs in the distal portion of the calf that became the standard in treating venous ulcers for several decades. Multiple modifications of Linton’s original technique were proposed to decrease wound complications and to avoid incisions too close to the ulcer. This led to a minimally invasive approach, pioneered by DePalma and Edwards, and culminated in the use of endoscopy, first described by Hauer. Adding a tourniquet and gas insufflation, Gloviczki and Conrad transformed subfascial endoscopic perforating vein surgery (SEPS) into a reliable procedure that disseminated around the world and replaced the open surgical approach.
The more recent development of endovascular treatment options for reflux in superficial veins has established a new standard for patients with chronic venous insufficiency (CVI), in whom the invasiveness and risk for wound complications associated with SEPS exceed those of the treatment of saphenous veins. As a result, new modalities, such as ultrasound-guided percutaneous ablation of perforating veins (PAPS) with sclerosing solutions or thermal energy, have become increasingly popular.
Renewed interest in treatment outcomes of IPVs and their role in venous pathophysiology uncovered a lack of reliable information related to their impact on the natural history and severity of CVI. Despite a growing body of evidence substantiating decision making in the management of patients with CVI, the indications for eliminating PV reflux remain imprecise.
In this chapter we describe the role of IPVs in the pathogenesis and natural history of chronic venous disease (CVD), diagnostic techniques for identification of PVs, and criteria for assessing their incompetence. We discuss indications and techniques for the interruption of IPVs as well as the outcomes of these treatment modalities. We have made every attempt to stay within the framework of evidence-based medicine. However, because some or perhaps many aspects of the topic have not been subjected to proper scientific investigation, we refer to our individual and group experience when appropriate. We identify such statements because they introduce substantial bias.
Pathophysiology
The role of PVs in normal physiology and their role in the pathogenesis of CVD are discussed in detail in Chapters 11 and 12. In this chapter, we review only the relevant aspects of PVs to better understand the rationale for the treatment of IPVs.
Normal Perforating Veins
The venous system of the lower extremity is rich in interconnections between the individual vessels. Because of the numerous collateral circulatory routes established, disruption of venous flow as well as compression of the veins by contracting muscles during locomotion or external pressure is prevented during changes in position of the body and extremity itself. By crossing the proper fascia of the extremity, PVs provide a connection between the superficial and the deep venous systems in the calf and thigh. There are about 60 PVs in a lower extremity,3 and most if not all of them have valves directing flow from the superficial to the deep veins.
Our understanding of PV anatomy and normal physiology is based mainly on studies conducted during the 1960s and 1970s and on inferences from clinical observations of pathologic conditions. Although some anatomic studies demonstrated that all PVs have valves,4 other showed that some PVs are avalvular.3,5 The latter, however, can be found in only a minority of extremities and not more frequently than two veins per extremity.3 These anatomic data are consistent with findings from duplex scans of asymptomatic legs, only 3.6% of which have bidirectional flow in PVs.6 Although the hemodynamic role of PVs in normal circulation has not been addressed by experimental or direct observational studies, it is unlikely that the current theory could be meaningfully challenged. Whether PVs provide collateral ways for blood flow to bypass areas of obstruction of the superficial veins in situations such as compression from outside during sitting or whether their presence facilitates outflow from superficial veins when pressure in the deep veins drops during walking, or both, it is clear that flow in healthy PVs is directed from the superficial into the deep system.
The role and significance of IPVs are much less obvious. In the absence of functioning valves, blood flow in a PV is governed mainly by the pressure gradient in adjoined segments of the superficial and deep systems. Active contraction of the venous wall and compression by surrounding muscles cannot compensate for the absence of the valve, especially in enlarged PVs with significantly degenerated walls. Therefore, knowledge of the hemodynamic function of the deep and superficial systems is critical for understanding the role of IPVs, and this role may differ according to the status of each of the two systems.
Hemodynamic Impact of Perforating Vein Incompetence
IPVs can be found in 40% to 60% of extremities with primary CVD and even more frequently in legs with postthrombotic disease.7–9 Isolated incompetence of a PV is extremely rare,10,11 and in most cases IPVs coexist with insufficiency of the superficial veins, deep veins, or both. Although the prevalence of IPVs is slightly higher in legs with both deep and superficial reflux than in limbs with only superficial reflux,8 the presence of axial superficial reflux appears to be the major factor associated with a higher prevalence of IPVs.9 More than 60% of extremities with axial reflux in the great saphenous vein (GSV) have incompetent PVs, as opposed to 38% of extremities with only segmental superficial reflux.7 An association between axial superficial reflux and PV incompetence has been shown during the progression of CVD. Within 1 or 2 years of observation, new refluxing segments in the superficial system develop in about a third of legs with varicose veins.12,13 In half of these cases, the new IPV also develops during the same time.12,14
The observation that correction of superficial reflux leads to resolution of perforator incompetence in most limbs supports the concept that superficial reflux causes an “overload” of PVs resulting in dilatation and incompetence.15 However, treatment of saphenous reflux by either stripping or ablation results in a significant increase in resistance to outward flow in PVs. Therefore, elimination of PV reflux after such treatment does not necessarily mean that the competence of the PV valves will be regained. The presence of these incompetent perforators without demonstrated reflux may be a reason that “isolated” IPVs are found in previously treated extremities and cause new symptoms and worsening hemodynamics.10,16 In addition, surgical treatment of superficial veins often fails to reverse PV incompetence, and new IPVs develop in limbs previously treated surgically.14,17
The presence of deep reflux further complicates the situation. An IPV may cause reversal of flow in an adjoined segment of deep vein, which is sometimes mistakenly interpreted as deep venous insufficiency but really reflects flow from the segment of deep vein between competent valves through an incompetent perforator into a connected superficial vein. In these cases, correction of superficial insufficiency with or without IPV interruption leads to disappearance of reversed flow in the deep vein segment.18 When deep reflux is caused by true valvular incompetence, this reflux may persist after the treatment of superficial veins.19 Thus, interpretation of IPV function based on data from clinical studies is not always possible.
Direct measurement of blood flow in IPVs and venous pressure in superficial veins demonstrated that even when incompetent, a PV can function in a way similar to competent PVs. During standing there is no blood flow through the IPV, and during walking the net flow through the IPV is from superficial veins to deep veins despite the bidirectional nature of flow during muscle contractions.20
Occlusion of an IPV does not influence venous pressure in superficial veins, whereas occlusion of an incompetent GSV normalizes hypertension.20 In light of these findings, the concept of transmission of the higher blood pressure from deep into superficial veins via IPVs during ambulation becomes questionable. Indirectly, this was confirmed by the demonstration of independent superficial and deep venous pressure changes during ambulation.21
Clinical Correlations of Perforating Vein Incompetence
The presence of IPVs is just one of many factors that determine the progression to advanced stages or affect the functional status and quality of life of CVD patients. The specific role of IPVs in the natural history and pathogenesis of CVD is less clear. The concept that IPVs can cause symptoms such as swelling and skin changes led to the development of and remains the justification for aggressive approaches to their treatment.22 Although a significant proportion of limbs with the most severe combination of deep, superficial, and perforator incompetence have no swelling or skin changes,23 IPVs alone can cause symptoms, affect hemodynamics, and increase the severity of disease.10,16 Even more convincing is the fact that successful treatment of an “isolated” IPV is associated with relief of symptoms and improvement of severity scores. In addition to their role in disease progression, IPVs can be a source of recurrent varices after treatment. This concept is supported by consistent findings of a high prevalence of IPVs in extremities with recurrent varices.24–27 Whether this association is important only in the presence of deep reflux or also plays a role in extremities with a competent deep system remains to be investigated.26,28
The relationship between IPVs and skin ulcers in the leg was historically the first to be demonstrated and was consistently confirmed in multiple small case series in which it was shown that surgical interruption of IPVs promotes healing and prevents recurrence of venous ulcers.29 This effect is especially prominent in primary CVD and less so in limbs with postthrombotic disease.30 Even treating IPVs alone can promote healing of ulcers in the majority of limbs with primary CVD.10 Large randomized controlled trials, however, failed to confirm this effect of IPV treatment. The Dutch Ulcer Trial, for example, showed ulcer healing rates of 83% in the surgical group and 73% in the group of patients treated by compression. Ulcer recurrence was almost the same in the two groups: 22% in the surgical group and 23% in the compression group.31 The results of this and other trials14,32,33 should be interpreted cautiously because their designs are far from ideal to answer the question of the role of IPVs in ulceration.
In a systematic review of the literature, O’Donnell showed that there is no level 1 evidence that treatment of IPVs alone affects ulcer healing or recurrence; level 1 evidence is available only for ligation of the GSV alone for decreasing recurrence of venous ulcers.34 He concluded that there is a need for randomized controlled trials to prove the role of IPV treatment in CEAP classes C5 and C6 disease.
Given the uncertainty of the physiologic role of IPVs and the lack of high-level evidence of efficacy of IPV interruptions, making a decision about IPV treatment is a challenging task. The relatively low risk of IPV interruption procedures may be the reason that they have been used in the past for the following indications: (1) normalization of venous hemodynamics, (2) prevention of the progression of CVD to more advanced stages, (3) resolution of symptoms and thereby a decrease in disease severity, (4) prevention of recurrent varicose veins, (5) promotion of ulcer healing, and (6) prevention of recurrent venous ulcers. Clear identification of one or more of these six points as a treatment goal in a patient or as an outcome to be measured in a study may facilitate our progress toward better understanding of the role that IPVs play in limbs with CVD.
Anatomy
As their name suggests, PVs perforate the deep fascia of the leg that separates the superficial and deep compartments; they can be classified as direct or indirect. Direct perforators connect the superficial to the deep venous systems, whereas indirect perforators join the venous sinuses of the calf muscles. Furthermore, PVs connect to each other via communicating veins above and underneath the deep muscle fascia. The majority of PVs are accompanied by perforating arteries (Fig. 59-1) and nerves that provide blood supply and innervation to the skin.3,35 Within the fascial orifice the artery is usually located proximal to the vein, but the topography of the subfascial and suprafascial segments of perforator arteries varies significantly. These arteries can be clearly identified by duplex ultrasound, and their position should be taken into account, especially if sclerotherapy is being considered as a treatment option.36
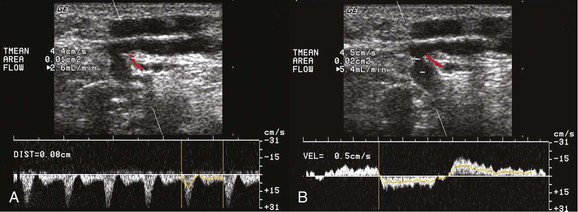
Figure 59-1 A, A small perforating artery is located next to the perforating vein at the fascial level. Pulsed wave Doppler confirms arterial flow. B, Doppler signal from the incompetent perforating vein indicates bidirectional flow.
The International Interdisciplinary Consensus Committee on Venous Anatomical Terminology recommends classifying PVs into six groups according to the segment of the lower extremity in which they are found37:
Perforators of the foot (venae perforantes pedis)
Perforators of the ankle (venae perforantes tarsalis)
Perforators of the leg (venae perforantes cruris)
Perforators of the knee (venae perforantes genus)
Perforators of the thigh (venae perforantes femoris)
Perforators of the gluteal muscles (venae perforantes glutealis)
Each group includes several subgroups (Fig. 59-2). One of the aims of this classification is to allow topographic description of PVs to avoid the use of personal names, which are often historically inaccurate.
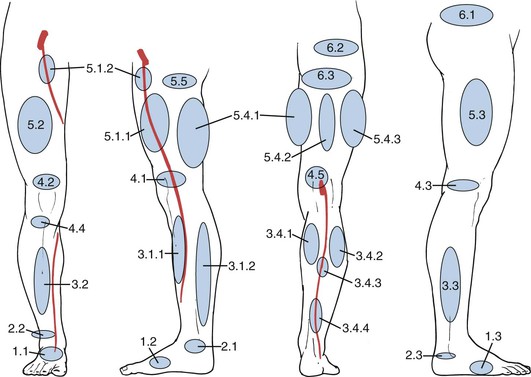
Figure 59-2 Schematic representation of the topography of the main groups of perforating veins (PVs). Foot PVs: 1.1, dorsal foot PV; 1.2, medial foot PV; 1.3, lateral foot PV. Ankle PVs: 2.1, medial ankle PV; 2.2, anterior ankle PV; 2.3, lateral ankle PV. Leg PVs: 3.1.1, paratibial PV; 3.1.2, posterior tibial PV; 3.2, anterior leg PV; 3.3, lateral leg PV; 3.4.1, medial gastrocnemius PV; 3.4.2, lateral gastrocnemius PV; 3.4.3, intergemellar PV; 3.4.4, para-achillean PV. Knee PVs: 4.1, medial knee PV; 4.2, suprapatellar PV; 4.3, lateral knee PV; 4.4, infrapatellar PV; 4.5, popliteal fossa PV. Thigh PVs: 5.1.1, PV of the femoral canal; 5.1.2, inguinal PV; 5.2, anterior thigh PV; 5.3, lateral thigh PV; 5.4.1, posteromedial thigh PV; 5.4.2, sciatic PV; 5.4.3, posterolateral thigh PV; 5.5, pudendal PV. Gluteal PVs: 6.1, superior gluteal PV; 6.2, midgluteal PV; 6.3, lower gluteal PV. (Redrawn from Caggiati A, et al; International Interdisciplinary Consensus Committee on Venous Anatomical Terminology: Nomenclature of the veins of the lower limbs: an international interdisciplinary consensus statement. J Vasc Surg 36:416-422, 2002.)
Many PVs are small vessels and have little clinical significance. The most important perforators from a clinical standpoint are the direct medial calf perforators, which cross the superficial posterior compartment. The posterior tibial PVs originate from the posterior accessory saphenous vein of the calf (posterior arch vein in the old terminology). The most distal posterior tibial perforators are located behind the medial malleolus, whereas the middle and upper posterior tibial perforators are located more proximally in the calf (at 7 to 9 cm and 10 to 12 cm from the medial malleolus, respectively) and about 1 inch medial to the tibia; these PVs connect the posterior arch vein to the posterior tibial veins (Cockett perforators). More proximal direct PVs are the paratibial direct perforators or “24-cm perforators,” which are located closer to the tibia and 18 to 22 cm from the medial malleolus, as evident in anatomic cadaveric studies (Fig. 59-3). Another group of medial calf perforators, found just below the knee, is known as Boyd’s perforators. They connect the GSV and its tributaries to the tibial or popliteal veins. Also of probable clinical importance are the posterolateral or peroneal perforators, which connect tributaries of the short saphenous vein to the peroneal veins. Among these, the most important are Bassi’s perforator, located at 5 to 7 cm, and the “12-cm perforator,” located at 12 to 14 cm from the lateral aspect of the ankle. Thigh perforators are less developed than calf PVs. The main ones are Dodd’s perforators and the hunterian perforators, which are located in the medial aspect of the thigh and connect the GSV to the popliteal or femoral veins. Other PVs connect the superficial system to the profunda femoris vein.
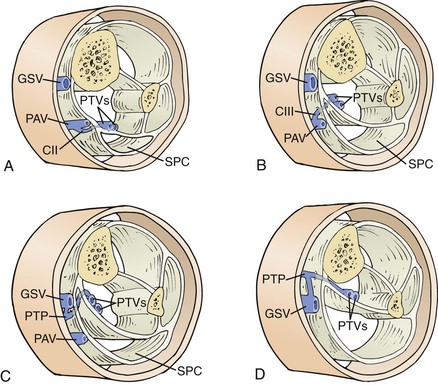
Figure 59-3 Compartments and medial veins of the leg. Cross-sections are shown at the level of Cockett II (posterior tibial perforator) (A), Cockett III (B), “24 cm” (C), and more proximal paratibial (D) perforating veins. CII, Cockett II; CIII, Cockett III; GSV, great saphenous vein; PAV, posterior arch vein; PTP, paratibial perforator; PTVs, posterior tibial veins; SPC, superficial posterior compartment. (From Gloviczki P, Bergan J, editors: Atlas of endoscopic perforator vein surgery, London, 1998, Springer-Verlag.)
Diagnostic Evaluation
Clinical Investigation (CEAP Level 1)
Large IPVs can be identified by palpation of the fascial defect, especially when varicose veins are located in the same area. By applying gentle pressure against this defect, an investigator can feel retrograde flow when a patient performs the Valsalva maneuver or coughs. Handheld continuous wave Doppler ultrasonography can be the next diagnostic step, but its sensitivity is insufficient and its specificity is not different from that of physical examination, which is close to zero.38
The best test to identify IPVs is the duplex ultrasound (see Chapter 18). This test should be performed in all patients with CVD. The invasive nature of venography, its questionable diagnostic properties for identification of IPVs,38 and its inability to map the exact location of IPVs make its use in CVD limited to selected cases needing venous reconstruction.
Noninvasive Vascular Investigation (CEAP Level 2)
The size of PVs and their hemodynamic properties are to a great extent affected by the position of the body. Most authors recommend performing the scan while the patient is standing or sitting; however, the 30-degree head-up supine position gives similar results.39 All surfaces of the extremity should be carefully scanned. The location of IPVs varies, and indirect IPVs can be found at a distance from major axial veins.
Because most PVs are rather short veins, induction of reversed flow in them is a great challenge. To initiate a reversed flow of appropriate duration, the connected superficial vein should be almost empty and the adjoined deep vein should contain a sufficient volume of blood. In many cases this is difficult to achieve, especially when the test is performed on a standing patient. To solve this problem, two sets of criteria for PV incompetence were suggested: a shorter time cut-point of 0.35 second to define the reflux12 and the use of PV diameter instead of reflux time.40 Diameter should be measured on a B-mode transverse section at the site where the vein penetrates the fascia (Fig. 59-4). Reflux time should be measured on a longitudinal section using a pulsed wave mode combined with simultaneous B-mode (duplex) or with the addition of a color Doppler mode (triplex). The site for measurement of reflux time should be the same as that for measurement of diameter—at the level of the fascial orifice (Fig. 59-5).
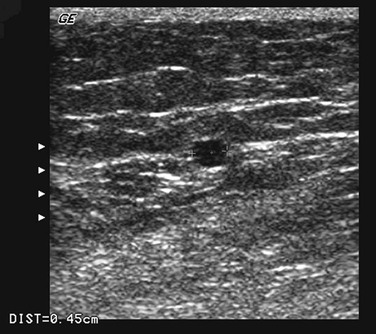
Figure 59-4 Measurement of perforating vein (PV) diameter is performed in a B-mode transverse section of the PV at the level of the fascial orifice.
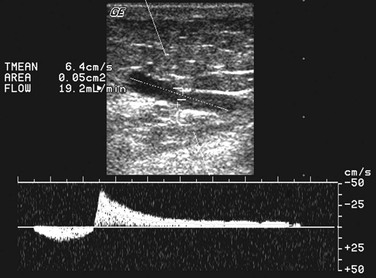
Figure 59-5 Measurement of flow velocity in a perforating vein (PV) is performed in Duplex mode in a longitudinal section of the PV at the level of the fascial orifice.
In our experience, using both approaches simultaneously has proved beneficial. Small PVs less than 2 mm in diameter have questionable clinical significance, even when reversed flow through them exceeds the cut-point for reflux. They also present a technical challenge for treatment and often undergo spontaneous thrombosis and resolution after ablation of the connected segment of superficial vein. PVs larger than 4 mm in diameter are almost certainly pathologic and may be identified as incompetent even when the reversed flow through them is shorter than the cut-point for reflux. Finally, PVs 2 to 4 mm in diameter should be considered incompetent only when they meet the appropriate hemodynamic criteria.
When treatment of an IPV is being considered, duplex scanning should be repeated in the exact position as the patient will be in during the treatment. This can be supine, prone, or on the side, depending on the selected access point. Shifting of anatomic structures can markedly affect the topographic relationship between subfascial and suprafascial segments of the IPV and superficial veins, which in turn will change the position of a desirable access point.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


