Chapter 55
Chronic Venous Disorders
General Considerations
Joseph D. Raffetto, Robert T. Eberhardt
Based on a chapter in the seventh edition by Joseph D. Raffetto and Robert T. Eberhardt
Chronic venous disorders encompass a spectrum of venous diseases from simple telangiectases (spider veins) and reticular veins, varicose veins, leg edema from dysfunctional venous tone with valve incompetence and abnormal calf muscle pump function, to more severe and advanced forms of venous disorders, including hyperpigmented skin changes, dermal sclerosis, and ulcer formation. Part of the spectrum of chronic venous disorders includes varicose veins, edema, and skin changes and ulcers affecting the lower limb, which are categorized as chronic venous disease (CVD).
Chronic venous disorders with manifestations specific to abnormal venous function are termed chronic venous insufficiency (CVI). A distinguishing feature of CVD and CVI is that CVI indicates more advanced forms of chronic venous disorders. Accordingly, CVI includes manifestations such as skin pigmentation, venous eczema, lipodermatosclerosis, atrophie blanche, and healed or active ulcers. Use of these precise definitions is not universal inasmuch as other reports also consider venous edema early evidence of CVI.
CVD is a very common problem, with varicose veins affecting more than 25 million adults in the United States, with more than 6 million having more advanced venous disease.1 Because of the high prevalence of venous disease, the National Venous Screening Program was conducted by the American Venous Forum (AVF) in the United States to increase awareness. The program identified varicose veins in more than 30% of participants and more advanced venous disease in more than 10%.2
Varicose veins have been recognized since the advent of recorded history, and manifestations of CVI, including edema and ulceration, since biblical times. The use of compression therapy dates back to Roman times, with foot soldiers using tight wraps to reduce the discomfort induced by prolonged standing. However, our modern understanding of the pathophysiology of CVI did not arise until the work of Brodie and Trendelenburg in the 1850s and 1890s describing superficial and deep venous reflux. Trendelenburg was credited with introducing surgery for varicose veins, thus marking the beginning of modern vascular surgery for this problem. There was an early focus on removal of varicose veins or their main trunks, and Keller, Mayo, and Babcock described various methods for stripping the saphenous vein in the early 1900s. Around this time, the German dermatologist Unna developed a paste gauze compression dressing that remains widely used today for the treatment of venous ulcers. Sclerotherapy for varicose veins was introduced by McPheeters and Dixon in the 1920s and was used extensively for the next 2 decades. Linton’s description of ambulatory venous hypertension in the 1950s furthered our pathophysiologic understanding of CVI. He also described the perforating veins of the leg and a surgical procedure for perforator interruption. Improvements in compression therapy arose from the work of the engineer Conrad Jobst, who introduced graduated compression stockings in an effort to mimic the beneficial actions of limb submersion in pool water. Finally, efforts to correct incompetence in the deep venous system were initiated in the 1970s and 1980s with clinical descriptions of valvular reconstruction by Kistner and vein valve transplantation by Taheri.
Epidemiology
CVD, including varicose veins and CVI, is an extremely common medical condition that has a significant impact on an individual’s health and the health care system.1 Estimates of the prevalence of CVD vary because of differences in the classification or definition used, methods of evaluation, and geographic regions studied.
The most common estimates of the prevalence of varicose veins have been between 5% and 30% in the adult population, but reports have ranged from less than 1% to greater than 70%.1 In an effort to assess the global prevalence of CVD, the Vein Consult Program was initiated, in which trained general practitioners screened for CVD. The study evaluated more than 91,000 participants in various countries (Western Europe, Central and Eastern Europe, Latin America, Middle East, Far East), and found a worldwide prevalence of clinically significant CVD of 63.9%.3 The prevalence of varicose veins was higher in developed, industrial countries than in underdeveloped countries. The San Valentino Vascular Screening Project from Italy found a 7% prevalence of varicose veins in 30,000 participants evaluated by clinical assessment and duplex ultrasound.4 The incident rate for the development of varicose veins could be estimated from the Framingham study, which reported an annual incidence of 2.6% in women and 1.9% in men.5 Most studies have found a greater prevalence of varicose veins in women, with an approximate twofold predominance, although this has not been universal. The findings of the San Diego Population Study supported this concept, with varicose veins being observed in 28% of women and 15% of men.6 Similarly, in the National Venous Screening Program, uncomplicated varicose veins were seen in 23% of the participants, of whom 77% were women.2 In a large French population-based study involving 4 regions with 8000 participants, varicose veins were found in 50.5% of women versus 30.1% of men (P < .001).7 Varicose veins were also more frequent in women in the Vein Consult Program.3 In contrast, other studies, including the Edinburgh Vein Study, reported a greater prevalence of varicose veins in men than in women (40% vs 32%).8 A recently published study from the Edinburgh Vein Study, with a mean follow-up of 13.4 years, found that the overall incidence of varicose veins was 18.2%, with the 13-year, age-adjusted incidence similar in men and women (15.2% vs 17.4%; P = .97).9 The apparent female predilection has been presumed to be due to the effect of pregnancy and possibly hormonal influences. Numerous other risk factors are associated with the development of varicose veins, including older age, family history in both sexes, obesity, a history of phlebitis, and a standing occupation.9 Female sex was a significant risk factor for development of nonsaphenous varicose veins.7 Additional behavioral factors such as smoking, physical inactivity, and low-fiber diets have also been suggested to play a role. There may also be ethnic differences, with the San Diego Population Study finding varicose veins to be most prevalent in Hispanics (26%) and least prevalent in Asians (19%).6
Prevalence estimates for CVI depend on the inclusion or exclusion of specific clinical features and on the methods used to confirm the diagnosis. Studies considering only more advanced manifestations of CVI, such as skin changes, will have a lower occurrence than studies that include manifestations such as edema. In addition, use of imaging methods to establish a diagnosis of CVI will influence the prevalence estimates. The San Diego Population Study found visible trophic changes attributed to CVD in 6.2% of the overall cohort compared with abnormal duplex findings in 27.9%.6 The National Venous Screening Program found 9% of participants had venous disease with advanced skin changes.2 Varicose veins, age, and pitting edema were the most significant risk factors for trophic skin changes seen in the French population-based study.7 Age is another important risk factor for CVI. The Edinburgh Vein Study found that the incidence of venous reflux on screening duplex ultrasound rose significantly with age.10 In the Bonn Vein Study, a population-based, cross-sectional study of 3072 participants, found reflux in 35.3% of patients. The prevalence of reflux in the superficial veins was more common in women, whereas reflux in the deep veins was more common in men, superficial reflux prevalence increased with age and higher clinical stages.11 In contrast to varicose veins, there appears to be no significant sex difference for CVI or perhaps a slight male preponderance. The rate of CVI did not differ between men and women in the Vein Consult Program.3 This was also supported by other studies in which the incidence of CVI was similar in men and women, and the CVI incidence increased with age.9 The Edinburgh Vein Study found venous reflux present in 9.4% of men and 6.6% of women using screening duplex ultrasound.10 Many other risk factors associated with CVI, not surprisingly, are similar to those for varicose veins, including a family history of varicose veins, obesity or large waist circumference, high number of pregnancies, a history of phlebitis, and prolonged standing.1,12,13 There may be additional factors more strongly associated with CVI, such as previous leg injury and physical inactivity.12,14 Another difference may reside in the ethnic predisposition for CVI, with more advanced disease being seen more commonly in non-Hispanic whites and less commonly in African Americans.6,13
The most serious consequences of CVI, namely, venous ulcers, either active or healed, are seen in approximately 1% of the adult population.1 Risk factors associated with the first-time development of a venous ulcer include maternal family history, physical activity, and history of deep venous thrombosis.15 It is estimated that approximately 5 million people in the United States have CVI and approximately 500,000 have chronic venous ulcers.16 The overall prognosis of venous ulcers is poor, with delayed healing and recurrent ulceration being very common.17 A majority of venous ulcers will require prolonged therapy, often lasting longer than a year.18
CVD has a significant impact on health care resources. Millions of people seek medical attention annually for varicose veins because of their cosmetic appearance or associated symptoms. Although often minimized, the cosmetic consequences may adversely affect an individual’s quality of life (QoL). An even greater socioeconomic impact is seen with more advanced venous disease. Venous ulceration has dramatic consequences that impair an individual’s ability to engage in social and occupational activities, reduces QoL, and imposes financial constraints. The disability resulting from venous ulcers leads to loss of productive work hours, estimated at two million workdays per year, and may cause early retirement, found in up to 12.5% of workers with venous ulcers.19 There is a significant financial burden of CVD on the health care system, with an estimated $3 billion per year being spent on the treatment of venous wounds in the United States.20 In countries with developed health care systems, CVD and especially venous ulcers, require considerable resources and have been estimated to account for up to 1% to 2% of the total health care budget.21,22
Pathogenesis
Macrocirculatory Dysfunction
Venous pathology develops when venous pressure is increased and return of blood is impaired through several mechanisms (see Chapters 11 and 12),23 including valvular incompetence of the axial deep or superficial veins, perforator valve incompetence, venous obstruction, or a combination of these processes. Muscle pump dysfunction also contributes to venous pathology. These mechanisms induce venous hypertension, particularly with standing or ambulation (Fig. 55-1).
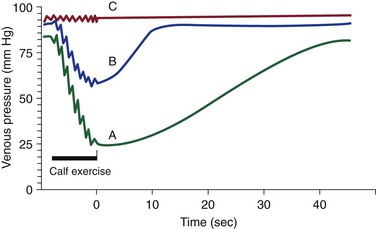
Figure 55-1 Foot venous pressure during calf exercise and rest over time in the standing position. Curve A shows normal venous pressure. Resting standing venous pressure is 80 to 90 mm Hg. With calf exercise the pressure drops to 20 to 30 mm Hg, or greater than a 50% decrease. Return to resting pressure is gradual, with refill taking more than 20 seconds. Curve B shows abnormal venous pressure with venous reflux. Resting or standing venous pressure is usually higher than normal. The drop in pressure with exercise is blunted (<50% decrease). Return of venous pressure to the resting level is rapid with a short refill time (<20 seconds). Curve C shows abnormal venous pressure with venous obstruction. Resting or standing venous pressure is usually higher than normal. There is minimal to no drop in pressure with exercise.
With valve failure of the deep veins, blood volume is pumped out of the extremity, but refill occurs by both arterial inflow and pathologic retrograde venous flow. Venous pressure immediately after ambulation remains relatively elevated, and veins refill very quickly with the development of high venous pressure in the absence of muscle contraction. Dysfunction of the valves of the deep system is most often a consequence of damage from previous deep venous thrombosis.24
Dysfunction or incompetence of the valves in the superficial venous system also allows retrograde flow of blood and the development of increased hydrostatic pressure. Valve failure may be primary and occur as a result of preexisting weakness in the vessel wall or valve leaflets or may be secondary to direct injury, superficial phlebitis, or excessive venous distention resulting from hormonal effects or high pressure.23 Failure of valves located at junctions of the deep and superficial systems, most notably at the saphenofemoral and saphenopopliteal junctions, allows high pressure to enter the superficial veins. The traditional view of this situation is that venous dilatation and varicose veins are thought to form and propagate from the junctional site down the extremity.
High pressure can also enter the superficial system because of failure of the valves in the communicating perforator veins.25,26 Perforator valve incompetence allows blood to flow from deep veins backward into the superficial system and allows transmission of the high pressures generated by the calf muscle pump. This local high pressure can produce excessive venous dilatation and secondary failure of superficial vein valves. As a result, a cluster of dilated veins develops at this site and leads to ascending venous reflux.25,26 This concept of ascending reflux is supported by other studies.27,28 As a consequence of these processes, reflux may occur in multiple venous segments, even in isolation.29
Reflux may also occur in venous tributaries in the absence of any axial or perforator reflux. Previous studies have found a 9.7% prevalence of tributary reflux in limbs, and of all tributaries studied, 19.9% had reflux. The most common tributaries with reflux were in communication with the great saphenous vein (65%), small saphenous vein (19%), or both (7%). Thus, reflux confined to the superficial tributaries occurs throughout the limb, and importantly, tributary reflux can develop in the absence of reflux in the truncal superficial or deep veins or perforator veins.30 This process of isolated tributary reflux may contribute to progression of disease within the other superficial or deep venous segments.
Obstruction of the deep veins may limit the outflow of blood and cause increased venous pressure with muscle contraction and secondary muscle pump dysfunction. Obstruction may occur as a result of an intrinsic venous process, such as previous deep venous thrombosis with inadequate recanalization or venous stenosis, or as a result of extrinsic compression, as in May-Thurner’s syndrome. In patients with postthrombotic syndrome, destruction of the valves results in secondary valvular incompetence, and chronic obstruction of deep veins from thrombosis causes persistent venous hypertension at rest and during ambulation. Venous outflow obstruction has a significant role in the pathogenesis of CVI and its clinical expression.31
Dysfunction of the muscle pumps leads to ineffective venous emptying from the extremity. Clinically significant muscle pump dysfunction often occurs with severe reflux or obstruction. The immediate postambulatory venous pressure will be nearly as high as the pressure after prolonged standing. Muscle pump dysfunction is a contributing factor to the development of venous insufficiency and complications of venous ulcers.32,33
Genetic Predisposition
Several studies have evaluated the potential genetic basis for the development of CVD, including varicose veins and venous ulcers. The likelihood for the development of varicose veins was evaluated in a prospective study of 67 patients and their parents. The patients’ nonaffected spouses and parents were used as controls, for a total of 402 participants. The risk of varicose veins’ developing was 90% if both parents were affected, 25% for males and 62% for females if one parent was affected, and 20% if neither parent was affected. These data suggest an autosomal dominant gene with variable penetrance. The lower incidence in males with an affected parent and the spontaneous development in patients without affected parents suggest that males may be more resistant to varicose vein formation, and that other factors exist.34
Although the specific gene causing CVD is unknown, there are several interesting studies supporting a genetic cause. In the rare primary lymphedema–distichiasis syndrome, the gene mutation FOXC2 is commonly associated with varicose veins at an early age as one of its phenotypic features. These findings have implicated the FOXC2 gene in the pathogenesis of varicose vein formation and provided evidence that the development of varicose veins has a heritable element.35,36 In the cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) pedigree with varicose veins, a heterozygous mutation of the Notch3 gene has been identified and is thought to lead to degeneration of venous vascular smooth muscle cells.37 In addition, gene expression profiles on varicose veins have shown upregulation of 82 genes from a microarray of 3063 human cDNA sequences. Of interest, genes regulating production of the extracellular matrix, cytoskeletal proteins, and myofibroblasts were upregulated in varicose veins.38 Collagen vascular diseases are systemic disorders with either collagen or elastin abnormalities. Ehlers-Danlos’ syndrome is due to abnormalities in collagen synthesis, and type IV is associated with varicose veins.39,40 In contrast, Marfan’s syndrome, caused by a defect in fibrillin-1, which is required for the synthesis of elastic fiber, does not appear to be associated with varicose veins.41
There may also be a genetic predisposition for the formation of venous ulcers. Studies have evaluated the role of iron metabolism and fibrin cross-linking in ulcer pathogenesis. CVD is associated with iron overload and hemosiderin deposition, free radical formation with tissue injury, and progression to skin ulcer formation.42,43 Factor XIII is an important cross-linking protein that plays a key role during ulcer healing.44 Based on these observations, the genes associated with hemochromatosis and factor XIII were found to have mutations in patients with CVD. The hemochromatosis C282Y (HFE) gene mutation and certain factor XIII V34L gene variants were associated with an increased risk for more severe forms of CVD, including venous ulcers and a variable healing response to surgery.45–47 In an attempt to assess various candidate genes that could potentially provide prognostic information, DNA-array genotyping was assessed in 638 individuals by selecting several single nucleotide polymorphisms in candidate genes of HFE (C282Y, H63D), FPN1 (−8CG, ferroportin gene involved in iron metabolism), MMP12 (−82AG) and FXIII (V34L). The patients had 221 venous leg ulcers (171 primary and 50 secondary), 112 patients had edema and skin changes, and 305 were matched healthy controls. The important findings in this study were that the FPN1-8GG genotype had an overall CVD risk of 4.3 and a venous ulcer risk of 5.2, and was similar for patients with primary disease and venous ulcers. In addition, the MMP12-82AA genotype also had a venous ulcer risk of 1.96 for only primary disease, but the MMP12 GG-genotype was associated with significantly smaller venous ulcers. These data need to be validated in prospective studies, but offer important information on the genetic aspects of CVD, with implications for prognosis and treatment.48
There are several well-described genetic disorders involving chromosomal defects, characterized by venous hypertension secondary to venous-valvular aplasia. Klippel-Trenaunay’s syndrome is a well-described syndrome manifested at a young age with features of varicose veins, limb hypertrophy, and dermal capillary hemangiomas (port-wine stains). Congenital venous anomalies include atresia and agenesis of the deep venous system, valvular insufficiency, venous aneurysms, and the presence of embryonic veins. Additional abnormalities of the lymphatic system are often present.49,50 Another form of congenital venous disease is Parke-Weber’s syndrome, which has features of venous and lymphatic malformations, capillary malformations, and arteriovenous fistulae. It is associated with significant muscle pump dysfunction, valvular incompetence, and in later stages, advanced CVI.51
Venous Microcirculatory Disturbances and Dysfunction
Contributing to the macrocirculatory hemodynamic disturbances are alterations in the microcirculation (see Chapter 12).52,53 Changes in the hemodynamics of the large veins of the lower extremity are transmitted to the microcirculation and lead to venous microangiopathy.52 In addition, dysfunction of the microvenous valves seems to play a key role and may occur independently of the macrovenous dysfunction.54 The importance of this latter finding is that this is the first study to investigate the superficial microvenous valves, and associate the degree of microvenous valve level insufficiency in the microcirculation and the saphenous venous system with the development of severe skin changes. Features of microangiopathy include elongation, dilatation, and tortuosity of capillary beds; thickening of basement membranes with increased collagen and elastic fibers; endothelial damage with widening of interendothelial spaces; however, interendothelial junctions have also been found to be normal in biopsies of advanced CVD patients, and increased pericapillary edema with “halo” formation. The increased permeability and high venous pressure of the abnormal capillaries lead to the accumulation of fluid, macromolecules, and extravasated red blood cells in the interstitial space. In addition to changes in blood vessels and connective tissue, alteration of the lymphatic network and nervous system may also occur. Fragmentation and destruction of microlymphatics may further impair drainage from the extremity, and dysfunction of local nerve fibers may alter regulatory mechanisms.
Several mechanisms have been postulated for the development of venous microangiopathy, including fibrin cuff formation, white blood cell trapping, and growth factor trapping.55–57 The fibrin cuff theory involves the accumulation of fluid containing fibrin in the pericapillary space. This cuff with impaired fibrinolysis is speculated to increase the diffusion barrier, inhibit the repair process, and maintain the inflammatory process. Another theory involves trapping of white blood cells in the capillaries (or postcapillary venules) with activation of leukocytes and inflammation. A related mechanism is trapping of growth factors, which makes these growth factors unavailable to facilitate healing. The pathophysiology is discussed in detail in Chapter 12.
Clinical Manifestations
History and Physical Examination
The most common signs and symptoms of venous disorders are related to the appearance of telangiectases, reticular veins, and varicose veins. With progressive dilatation and tortuosity, varicose veins may become painful as a result of venous distention. These veins are also prone to the development of superficial thrombophlebitis and occasional bleeding from the superficial veins and thinning of the overlying skin. Common symptoms related to CVD seen by the vascular specialist are pain, swelling, and ulceration of the legs. The pain or discomfort of the leg is typically described as heaviness or aching aggravated by prolonged standing and relieved by elevation. Additionally, chronic obstruction of the deep venous system may lead to venous claudication with intense leg cramping during ambulation because of exertion-induced venous hypertension. Edema often begins in the foot and ankle and extends up the leg with progressive worsening during the day as fluid accumulates in a dependent manner. The development of unilateral edema is suggestive of a venous etiology. Edema is thought to produce discomfort by increasing intracompartmental volume and pressure. Venous-related skin changes may also develop, including hyperpigmentation in the perimalleolar region secondary to hemosiderin deposition, lipodermatosclerosis with scarring and thickening of the skin secondary to fibrosis in the dermis and subcutaneous fatty tissue, and atrophie blanche characterized by circular whitish and atrophic skin surrounded by dilated capillaries and hyperpigmentation. There is also increased risk for other skin problems, such as eczematous dermatitis, cellulitis, and lymphangitis. Delayed healing of leg ulcers may occur and lead to protracted ulceration.58
A complete history, including medical and surgical history, is important in evaluating the etiology of edema and ulcerations as possible manifestations of CVI as supported by its Grade 1A recommendation in the Clinical Practice Guidelines by the Society for Vascular Surgery (SVS) and the AVF.59 These recommendations and the ones that follow in this chapter are from the AVF Venous Guidelines Committee and are based on the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) that assess the recommendation and quality for a given assessment and treatment. The recommendations are strong (Grade 1, benefits outweigh risks, burden, and costs) or weak (Grade 2, benefits are closely balanced but with less certainty with respect to the magnitude of benefit, risks, burden, and costs). The quality of evidence is strong (A, high-quality evidence from well-designed controlled trials, large center series), medium (B, moderate-quality evidence from controlled trials with design flaws, small center series), or low (C, low-quality evidence from case reports, expert opinion).59 The GRADE system will be used in this chapter where available data provides adequate information to render a recommendation. A history of deep venous thrombosis or phlebitis, use of anticoagulation, unexplained transient unilateral edema, or previous venous-related interventions favor a venous etiology. Similarly, a family history of varicosities, leg ulceration, or a thrombotic disorder supports a venous problem. A review of medications may reveal agents known to cause or contribute to leg edema. Other behavioral and environmental risk factors for CVD should be determined.
Physical examination is essential for the proper diagnosis and management of CVD (see Chapter 14). The venous examination should include an evaluation in both the supine and upright positions to induce maximal venous distention. The surface of the skin is examined for irregularities or bulges suggesting the presence of varicose veins. The distribution of varicose veins often follows the course of the affected superficial veins with dilatation of their branches. Palpation may reveal tenderness of the dilated veins. Other characteristic skin findings include hyperpigmentation, eczematous dermatitis, atrophie blanche, and lipodermatosclerosis. The edema is typically pitting, although before the development of overt edema there may be calf fullness or increased limb girth, so both calf muscle consistency and limb girth should be assessed. Protracted edema may become more resilient to palpation and is referred to as brawny edema. The appearance of a fan-shaped pattern of numerous intradermal veins on the medial or lateral aspect of the foot or ankle, termed corona phlebectatica (or inframalleolar ankle flare), is thought to be an early sign of advanced disease. The presence of active or healed ulcerations indicates advanced disease, and these ulcerations occur usually in the medial, and occasionally, in the lateral perimalleolar region at the site of major perforating veins and the greatest hydrostatic pressure.58
The examination for ulceration should include a detailed arterial evaluation and often a neurologic assessment for sensory deficits. A normal pulse essentially excludes arterial insufficiency; however, the ankle-brachial index may be necessary if pulses are masked by edema. Patients with diabetes mellitus often require a limited neurologic sensory examination to assess for peripheral neuropathy. Suspicious ulcers of prolonged duration with features suggestive of a mixed etiology should undergo biopsy to exclude malignancy.58
The classic Brodie-Trendelenburg test may be performed to help distinguish between deep and superficial reflux.60 The test is performed with the patient supine and the leg elevated to empty the veins. A tourniquet or manual compression is applied over the superficial veins before resuming the upright posture. In the presence of superficial venous disease, varicose veins will take more than 20 seconds to fill if compression is held cephalic to the point of reflux. Release of the tourniquet or manual compression allows rapid venous filling in the presence of superficial venous incompetence. With deep (or combined) venous insufficiency, the varicose veins appear rapidly despite use of the tourniquet or manual compression. The distribution of venous insufficiency may be assessed at the bedside with this technique. An additional maneuver may be performed as part of the Perthes test to assess the perforating veins. It involves application of a tourniquet as in the Brodie-Trendelenburg test, but walking is performed on assumption of the upright posture. Enlargement of varicosities below the tourniquet, as blood is forced into the superficial venous system with calf muscle contraction, is indicative of incompetent perforating veins.
Handheld continuous-wave Doppler ultrasound has been used to assist in bedside evaluation.61 The presence and direction of flow in veins, such as the common femoral vein, may be determined after maneuvers such as the Valsalva or manual compression of the thigh or calf. In the absence of venous insufficiency, minimal reversal of flow toward the feet should be detected with these maneuvers. Flow toward the feet lasting for more than 0.5 second is indicative of venous reflux. This technique has also been used to assess the great and small saphenous veins, although it is technically more difficult because the lack of direct visualization leaves one uncertain about the precise site of reflux.
Classification and Severity
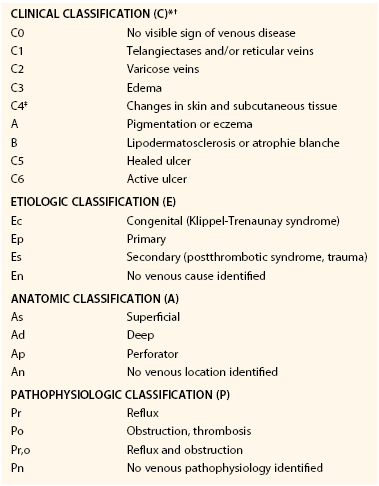
A consensus classification scheme was developed to standardize venous reporting for consistency in describing CVD.62 This classification, known as clinical, etiologic, anatomic, pathologic (CEAP), was adopted rapidly, but it had several limitations. The initial classification poorly defined telangiectases, reticular veins, and varicose veins, and was subjective, as well as subject to intraobserver or interobserver variability.63 Other investigators found an inadequate delineation of categories and significant heterogeneity.64 In recognition of these limitations, the CEAP classification underwent revision consisting of a basic (Table 55-1) and an advanced format, which are used in clinical practice and in research.65 The importance of CEAP is that it provides a method for consistent communication with specific descriptors, allows standardization of CVD into classes, and can be used to guide treatment and assess prognosis.
The use of CEAP, as reported by the Clinical Practice Guidelines, award a Grade 1A recommendation in the classification of patients with CVD.59 Because venous disease is dynamic, the advanced CEAP classification was constructed to allow a more complete assessment of CVD. Elements of the CEAP scheme include clinical class, etiology, anatomic, and pathophysiologic classifications. In the basic CEAP format, the highest clinical class is determined, whereas in advanced CEAP all levels of clinical class are described. The etiology for both basic and advanced CEAP has the same classification. The anatomic classification for basic CEAP provides descriptors for superficial, deep, and perforator disease. In the advanced CEAP scheme, the anatomic classification is categorized into 18 anatomic locations.65 The pathophysiology for both basic and advanced CEAP has the same classification. The CEAP classification may be used to reclassify CVD at any time because the classification may change with treatment.
Various investigative modalities are used to categorize the extent and severity of CVD as classified by CEAP. Although the history and physical examination will define nearly 90% of CVD patients, additional noninvasive and invasive tests can be performed to further delineate anatomic location and functionality. Therefore, the advanced CEAP classification has instituted three levels of investigation to characterize the diagnostic method used: level I includes office visits with history and physical examination; level II includes duplex ultrasound or plethysmography; and level III includes more invasive tests, such as venography, ambulatory venous pressure, and advanced imaging.
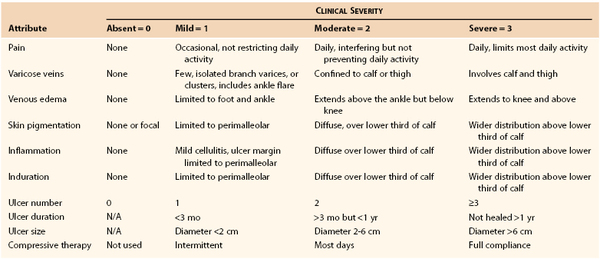
The Venous Severity Score (VSS) was developed to provide an objective measure of disease severity. It is an important tool for assessing longitudinal changes in CVD with treatment or when comparing different modalities of treatment. The VSS was designed to complement the CEAP classification by integrating elements from CEAP. Components of the VSS include the Venous Clinical Severity Score (VCSS), Venous Segmental Disease Score (VSDS), and Venous Disability Score (VDS).66 The VCSS is composed of 10 descriptors (pain, varicose veins, edema, pigmentation, inflammation, induration, number of ulcers, duration of ulcers, size of ulcers, compressive therapy) that escalate in severity with the increased area of the limb involved and are graded 0 to 3 (absent, mild, moderate, severe). To provide clarification of the terms, less ambiguity, and better definition of the descriptors, a revised VCSS was developed (Table 55-2).67 The original VCSS has been evaluated in clinical practice and validated as an important instrument for longitudinal research to assess outcomes after treatment with low variability.68 The VCSS has been demonstrated to increase with higher CEAP clinical class in a strong linear relationship.69 In addition, the VCSS has been recently validated with CIVIQ (see section on Clinical Outcomes Assessment: Quality of Life), CEAP, and ultrasound findings in the National Venous Screening Program. The correlation of different components of the VCSS varies with the venous assessment tool that is used, indicating the global application of the VCSS in determining overall severity of venous disease, while at the same time highlighting the strengths of the other venous assessment tools.70 The revised VCSS awaits trials for validation, but it is in current use. The Clinical Practice Guidelines award a Grade 1B recommendation when used in assessing patients with CVD and treatment outcomes.59 The VSDS evaluates the anatomic and pathophysiologic components of CEAP and has a maximum severity score of 10. The venous segments are graded according to reflux or obstruction. Reflux evaluates the common femoral vein to the calf veins and includes the superficial, perforator, and deep veins. Evaluation of obstruction also includes the inferior vena cava and iliac veins. The VDS is based on a score of 0 to 3 and takes into account the type of compression therapy, including leg elevation status, but does not include an element regarding impact on work hours. Both the VCSS and VDS are more sensitive than the CEAP clinical class to changes occurring with treatment.69,71
Clinical Outcomes Assessment: Quality of Life
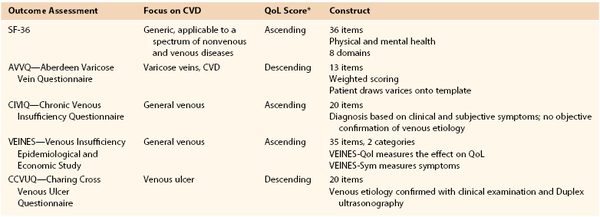
Clinical outcomes may be gauged by physician perception with use of the VSS or by patient perception in the form of generic and disease-specific QoL questionnaires (Table 55-3). Four important features that a QoL instrument must possess to be an effective tool for measuring outcomes are reliability, validity, responsiveness, and practicality.72 Reliability evaluates the consistency of the instrument in the answers provided by patients, and patients with similar conditions should answer questions in a similar way. Reliability is measured for internal consistency with Cronbach’s alpha, with a value greater than 0.7 indicating good reliability. Validity evaluates the ability of a question to measure the intended parameter. Responsiveness evaluates the ability of the instrument to detect important changes in health. Practicality evaluates the time necessary to complete the questionnaire, the completeness of the questionnaire, and patient acceptability and ease of administration.
The 36-item Short Form Health Survey (SF-36) is a generic QoL instrument widely used for a variety of pathologic conditions, including venous disease. The SF-36 evaluates both physical and mental health. The questionnaire has a score of 0 to 100, with higher scores indicating better health, and has been validated in CVD.73 The Aberdeen Varicose Vein Questionnaire (AVVQ) was designed to evaluate a surgical intervention for venous insufficiency. The AVVQ is a 13-question construct and is scored from 0 to 100, with lower scores indicating less severity. The AVVQ evaluates physical and social issues, including pain, edema, ulcers, skin discoloration, use of compression, and how the varicose veins affect overall well-being and function.72 The CIVIQ evaluates four areas of interest, including physical, psychological, social, and pain. The CIVIQ 2 is a revised version of the original CIVIQ that contains 20 equally weighted categories. The CIVIQ QoL was validated as a useful tool in assessing CVD,74 and was used as an outcome assessment after venous stenting for venous outflow obstruction.75 Another instrument evaluating CVD outcomes is the Venous Insufficiency Epidemiologic and Economic Study (VEINES) questionnaire.76 VEINES has two components, the QoL questionnaire (VEINES-Qol), which consists of 25 items focused on the effect of venous disease on QoL, and the symptoms questionnaire (VEINES-Sym), which consists of 10 items assessing venous symptoms. A decreasing VEINES score correlates with a higher CEAP clinical class. In addition, it has been found to be useful in assessing response to treatment, including ulcer healing, and in evaluating postthrombotic syndrome and its treatment.77,78 The Charing Cross Venous Ulcer Questionnaire was intended and validated against the SF-36 to assess venous ulcers. The Charing Cross Venous Ulcer Questionnaire consists of 20 items plus confirmation of the venous etiology of the ulcer with duplex ultrasound.79 The Clinical Practice Guidelines provide a Grade 1B for the use of disease-specific QoL assessment to evaluate patient-reported outcome and the severity of CVD.59
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree