24 Chronic Total Occlusion
 The Role of Percutaneous Coronary Intervention in Chronic Total Occlusions
The Role of Percutaneous Coronary Intervention in Chronic Total Occlusions
The initial “chronic” total occlusions tackled by Andreas Grüntzig, the pioneer of percutaneous coronary intervention (PCI), were those that had silently progressed from stenoses while the respective patients had been on the rather long waiting list for PCI typical for the late 1970s. The primary success rate was 62%.1 A “true” chronic total occlusion is usually defined as a 100% luminal diameter obstruction without flow in that segment of 3 months or more duration. In contrast to interventional cardiologists, cardiac surgeons may even prefer chronic occlusions to stenoses. Both require the same surgical technique, but the occluded coronary artery provides no competitive flow for the graft that might enhance its propensity for attrition. Moreover, an occluded native artery will usually not cause a major clinical problem should the graft occlude. The aspect of reduced risk in dealing with chronic occlusions pertains also to PCI. However, the heightened intricacy of recanalizing a chronic occlusion rather than dilating a stenosis impacts indications for PCI. Hence chronically occluded lesions account for 20% to 40% of patients with angiographically documented coronary artery disease, but they represent only 10% of targets for PCI. Chronic occlusions remain the single most important reason not to attempt PCI, thus favoring, instead, bypass surgery or medical treatment. It appears that the better the surgical program is developed, the less time is invested in recanalization attempts of chronic occlusions by PCI.
 Histology and Pathophysiology of Chronic Total Occlusion
Histology and Pathophysiology of Chronic Total Occlusion
Histology
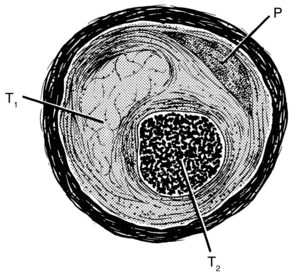
A chronic total coronary occlusion has several anatomical components.2,3 An atherosclerotic plaque is invariably present as a major or a minor part of the luminal obstruction. Thrombus is the complementary element. There may be a single clot of uniform structure and age or layers of clots of disparate structures and ages associated with fibrointimal proliferation. The latter situation signifies the occurrence of prior thrombi secondary to previous plaque fissures that may or may not have been totally occlusive. In cases in which they had been totally occlusive, these fissures were partially recanalized before subsequently reoccluding (Fig. 24-1). The most recent thrombus is assumed to obstruct the last lumen that had been patent up to the final complete occlusion of the particular coronary segment. The recanalization equipment should be passed through this thrombus. The texture of the thrombus is crucial for success or failure of PCI. The older and the more fibrosed and/or calcified a clot, the smaller the chance of crossing it safely. The point of entry to a total occlusion often comprises a thick, hard layer, called the proximal fibrous cap, making guidewire penetration potentially difficult. Similar but usually thinner is the distal fibrous cap, which can make the guidewire exit challenging after having progressed through the entire length of the occlusion. Spontaneous recanalization of a totally occluded segment may occur by lysis of a clot, development of several new channels through the thrombus (intraluminal microchannels), dilation of the adventitial vasa vasorum (bridging collaterals), or a combination of these mechanisms. Microchannels holding a mean diameter of only 0.007 inch are invisible on angiography. Angiographically, such a recanalization (functional occlusion) can be readily distinguished from a true total occlusion by the presence of antegrade flow, which may coexist with retrograde filling of the distal part of the vessel, as is easily demonstrable by angiography in case of ipsilateral collaterals. However, it is not possible to discern which of the aforementioned mechanisms is active for antegrade flow, and functional occlusion is difficult to differentiate from a subtotal stenosis. Tackling a subtotal stenosis that had never been completely occluded before and that shows no collateralization creates the risk of an acute infarction resulting from abrupt vessel closure; tackling a recanalized segment does not. Conversely, it is generally easy to pass a subtotal stenosis with a coronary guidewire, but it may be tedious or impossible, even with sophisticated equipment, to pass a recanalized segment, because the recanalization may consist of several tortuous microchannels in densely fibrosed tissue or be simulated by copious vasa vasorum.
Collaterals and Ischemic Symptoms
A total occlusion that is well collateralized is functionally equivalent to a 90% stenosis.4 It sustains myocardial viability but produces clinically apparent ischemia during periods of increased oxygen demand. Hence the patient with a chronic total coronary occlusion that was collateralized well enough at the time of the acute event to preserve part or all of the dependent myocardium is liable to have exertional angina. Such a patient may also have chest pain at rest because of increased oxygen demand secondary to spells of hypertension or tachycardia but will not face the major risk of unstable angina—i.e., acute occlusion with ensuing myocardial infarction.
 Percutaneous Coronary Intervention for Chronic Total Occlusion
Percutaneous Coronary Intervention for Chronic Total Occlusion
Improvement of clinical symptoms and normalization of a positive exercise test or restoration of vitality in the territory of the occluded vessel—together with a reasonable chance of technical success—provide the rationale and ethical basis for PCI in chronic total occlusion.5,6 Reduced left ventricular remodeling7 and improved survival8–13 have been observed after successful catheter-based recanalization. The most conspicuous benefit, however, consists in a significantly lower need for later coronary artery bypass grafting (CABG). Overall, the average left ventricular functional improvement after recanalization of chronic total coronary occlusions is not overwhelming and may escape detection by crude assessment. Moreover it is more likely to be found after recanalizing fairly recent occlusions. Finally, patients with untreated chronic total occlusions face a threefold risk of cardiac mortality or complications in case of future cardiac events.14 The case depicted in Figure 24-2 may be anecdotal, but it provides compelling evidence that in an individual case a recanalized artery may provide reverse collaterals to its former donor artery years later, thereby saving the patient’s life. These awaited benefits are predicated on persistent patency of the recanalized segment.
Open-Artery Hypothesis
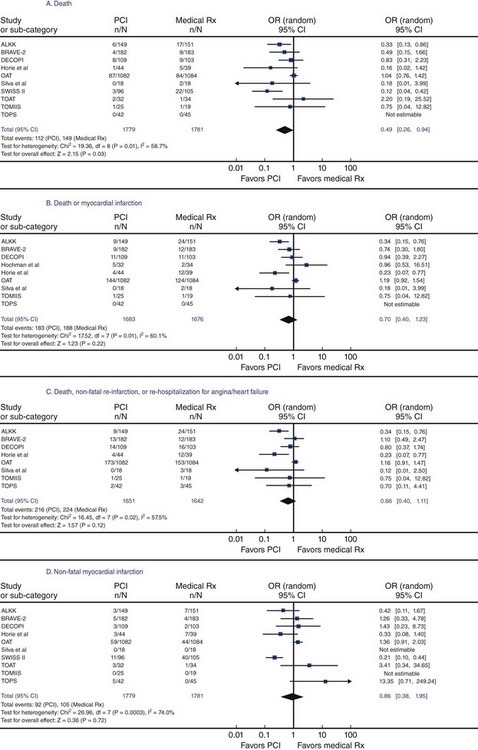
The debate is ongoing whether an open artery per se provides clinical benefit. This debate is partially ignited by mixing up study results examining different clinical scenarios. In this context, three distinct but heterogeneous clinical settings exist: first, opening an infarct-related artery within a few hours after symptom onset to salvage the jeopardized myocardium and prevent necrosis; second, opening an infarct-related artery several days to a few weeks after the appearance of symptoms to revert residual ischemia in the peri-infarct zone (the original open-artery hypothesis); and third, opening a chronically occluded artery (≥ 3 months occlusion age) that is collateralized and usually occluded without causing a myocardial infarction, in order to restore normal coronary blood flow to the supplied myocardium. Although the evidence supporting recanalization of an infarct-related artery in the acute setting is robust and ascertained, the optimal management strategy for the time beyond the first couple of days is mostly unclear. While previous studies denied a benefit from tackling these occlusions compared with medical treatment alone,15 common sense and more recent data suggest the contrary. The SWISSI II trial16 randomized patients within 3 months after myocardial infarction (provided that they had silent ischemia as verified by stress-test imaging and one- or two-vessel disease on angiography amenable for PCI) to either complete revascularization (96 patients) or intensive anti-ischemic drug treatment (105 patients). During a mean follow-up of 10.2 ± 2.6 years, 27 major adverse cardiac events (cardiac death, nonfatal myocardial infarction, or symptom-driven revascularization) occurred in the intervention group and 67 events occurred in the medical therapy group (adjusted hazard ratio [HR], 0.33; 95% confidence interval [CI], 0.20–0.55; P < 0.001), which corresponds with an absolute event reduction of 6.3% per year (95% CI, 3.7%-8.9%; P < 0.001). Left ventricular ejection fraction remained preserved in revascularized patients (mean of 54% ± 9.9% at baseline and 56% ± 8% at final follow-up) and decreased significantly (P < 0.001) in medically managed patients (mean of 60% ± 12% at baseline to 49% ± 8% at final follow-up). Abbate et al.17 performed a systematic review and metanalysis of randomized trials comparing PCI of the infarct-related artery with medical therapy in patients randomized >12 hours to 60 days after acute myocardial infarction. The survival and cardiac remodeling benefits in patients undergoing late PCI of the infarct-related artery are plotted in Figure 24-3. Data supporting a favorable impact of complete revascularization with PCI on survival in patients with at least one chronic total occlusion are accumulating.8–13 Figure 24-4 shows the influence of success in a recanalization attempt in three large registries with various follow-up periods. Procedure success has shown instrumental for longevity of patients with attempted revascularization of a chronic total occlusion (Table 24-1). Aziz et al.11 performed a retrospective study comparing the survival of patients with a successful percutaneous revascularization of a chronic total occlusion versus those with a failed procedure between the years 2000 and 2004. Technical success was 69%. During a mean follow-up of 1.7 ± 0.5 years, the mortality rate was 2.5% in the successfully recanalized patients and 7.3% in the failure group. The crude hazard ratio for death with recanalization failure was 3.92 (95% CI 1.56–10.07; P = 0.004). The rates of CABG were 3.2% versus 21.7% (P < 0.001) for success and failure patients, respectively. Multivariate analysis showed that recanalization failure was an independent predictor of death.
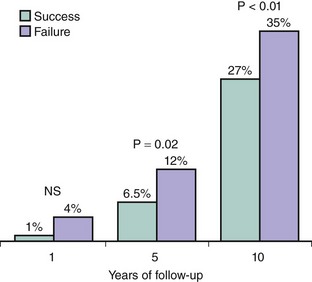
Figure 24-4 Mortality from three different registries with average follow-ups of 1 year (Total Occlusion Angioplasty Study of the Italian Society of Cardiology, TOAST-GISE with 286 successful and 83 failed recanalization attempts),8 5 years (Rotterdam Thoraxcenter experience with 576 successful and 309 failed recanalization attempts),9 and 10 years (Mid-American Heart Institute, Kansas City, Kansas, USA, with 1491 successful and 514 failed recanalization attempts).10 NS, not significant.
TABLE 24-1 Predictors of Long-Term Mortality after Attempted Revascularization of a Chronic Total Occlusion*
| Mid-american heart institute (2,013 patients)10 | |
| Success | 0.7 (0.5–0.8) |
| Age >70 years | 1.9 (1.5–2.4) |
| Ejection fraction <40% | 2.1 (1.7–2.7) |
| Double vessel disease | 1.5 (1.1–2.2) |
| Triple vessel disease | 1.9 (1.4–2.7) |
| Diabetes mellitus | 1.4 (1.1–1.8) |
| Creatinine >2.0 mg/dL | 2.2 (1.3–3.9) |
| Unstable angina | 1.3 (1.0–1.6) |
| Rotterdam thoraxcenter (874 patients)9 | |
| Success | 0.6 (0.3–1.0) |
| Age | 1.0 (1.0–1.1) |
| Multivessel disease | 4.3 (1.9–9.6) |
| Diabetes mellitus | 2.5 (1.3–4.7) |
* Hazard Ratio (95% confidence interval).
Prasad et al.12 examined the trends in procedural success and long-term outcomes after PCI for chronic total occlusions over 25 years from the Mayo Clinic registry. The patients were divided into four groups according to the time of their intervention: group 1 ( balloon angioplasty era), group 2 (early bailout stent era), group 3 (elective bare-metal stent era), and group 4 (drug-eluting stent era). Procedural success rates were 51%, 72%, 73%, and 70% (P < 0.001 for balloon angioplasty versus stent), respectively, in the four groups. During follow-up, the combined endpoint of death, myocardial infarction, or target lesion revascularization was significantly lower in the two most recent cohorts compared with those patients treated before (P = 0.001 for trend). Patients with a technical success did appear to have slightly greater survival starting at 6 years of follow-up, although technical failure was not an independent predictor of mortality in the multivariate analysis (HR 1.16 [95% CI 0.90–1.5], P = 0.25). Patients who underwent a failed recanalization were much more likely to be referred for surgical revascularization (11% vs. 1%). Valenti et al.13 sought to determine the impact on survival of successful drug-eluting stent-supported PCI for chronic total occlusion among 486 patients with 527 chronic total occlusions between 2003 and 2006. Recanalization of the targeted occlusion was successful in 71% of patients (68% of lesions). Multivessel intervention was performed in 62% of patients in whom recanalization of the chronic total occlusion was unsuccessful and in 71% of patients with successful recanalization of the chronic total occlusion (P = 0.062). At a median follow-up of 2 years (1.1–2.8 years), the cardiac survival rate was higher in the successfully recanalized group compared with the failure group (92 ± 2 vs. 87 ± 3%; P
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree