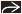Chronic Dyspnea
Christopher M. Walker, MD
DIFFERENTIAL DIAGNOSIS
Common
Pleural Effusion
Emphysema
Sarcoidosis
Bronchogenic Carcinoma
Less Common
Usual Interstitial Pneumonia
Nonspecific Interstitial Pneumonia
Respiratory Bronchiolitis-associated Interstitial Lung Disease
Radiation Pneumonitis
Mycobacterial Avium Complex
Lymphangitic Carcinomatosis
Pneumoconioses
Left to Right Shunt
Rare but Important
Bronchioloalveolar Cell Carcinoma
Constrictive Bronchiolitis
Lymphocytic Interstitial Pneumonia
Pulmonary Alveolar Proteinosis
Chronic Eosinophilic Pneumonia
Organizing Pneumonia
Lipoid Pneumonia
Langerhans Cell Histiocytosis
Lymphangiomyomatosis
Hypersensitivity Pneumonitis
Desquamative Interstitial Pneumonia
ESSENTIAL INFORMATION
Key Differential Diagnosis Issues
Review focuses on adult intrathoracic causes of dyspnea lasting weeks to years
Helpful Clues for Common Diagnoses
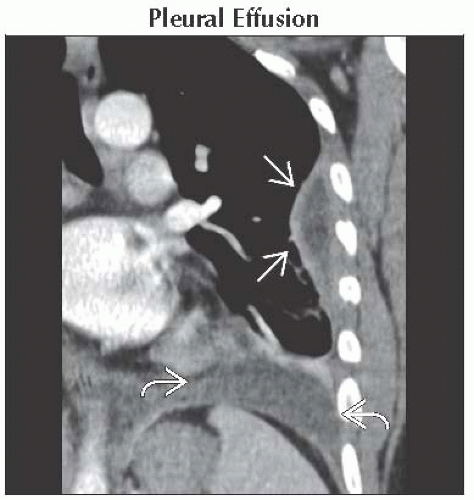
Pleural Effusion
Exudative effusions
Pleural thickening/enhancement in 60%
Infections, malignancy, connective tissue diseases, and asbestos exposure
Emphysema
Flat diaphragm and increased retrosternal clear space
Sarcoidosis
Symmetric right paratracheal, right hilar, and left hilar lymphadenopathy is called 1-2-3 sign or Garland triad
Perilymphatic lung nodules (nodules along fissures, subpleural lung, and bronchovascular bundles)
Bronchogenic Carcinoma
Nodule or mass in current/former smoker
Helpful Clues for Less Common Diagnoses
Usual Interstitial Pneumonia
Basal and subpleural fibrosis with honeycombing
± mediastinal lymphadenopathy
Most common cause is idiopathic pulmonary fibrosis
Nonspecific Interstitial Pneumonia
Associated with collagen vascular diseases
Lower lobe and peripheral ground-glass opacity
± subpleural sparing
Honeycombing rare
Respiratory Bronchiolitis-associated Interstitial Lung Disease
Symptomatic smoker
Upper lung centrilobular nodules of ground-glass opacity
Radiation Pneumonitis
1-4 months following radiation therapy
Ground-glass opacity with sharp borders
Disobeys anatomic boundaries
Mycobacterium Avium Complex
Older women
Middle lobe and lingular bronchiectasis
Tree in bud centrilobular opacities
Lymphangitic Carcinomatosis
Smooth or nodular thickening of interlobular septa
± hilar or mediastinal lymphadenopathy
± pleural effusion
Pneumoconioses
Asbestosis
Posterobasal and subpleural lung
Bilateral pleural plaques
Honeycombing and thickened septa late
Silicosis and coal worker’s pneumoconiosis
Posterior upper lung predominant
Centrilobular and subpleural nodules
Nodules may coalesce to form progressive massive fibrosis
Left to Right Shunt
ASD and partial anomalous pulmonary venous return are most common etiologies in adults
Helpful Clues for Rare Diagnoses
Bronchioloalveolar Cell Carcinoma
Most common presentation
Solitary pulmonary nodule
Chronic ground-glass opacity
± “pseudocavitation” with cystic spaces
Constrictive Bronchiolitis
Synonyms
Bronchiolitis obliterans or obliterative bronchiolitis
Causes include
Infection, toxic fume inhalation, collagen vascular diseases, and chronic rejection
Bronchiectasis, mosaic perfusion, and expiratory air-trapping
Lymphocytic Interstitial Pneumonia
Strong association with Sjögren syndrome
AIDS defining in children
Ground-glass opacity and nodules ± isolated or diffuse lung cysts
Pulmonary Alveolar Proteinosis
Crazy-paving pattern
Geographic bilateral ground-glass opacities with interlobular septal thickening
Idiopathic or seen with silicosis, malignancy, and chemotherapeutic medications
Exclude acute causes of crazy-paving, such as ARDS by history
Chronic Eosinophilic Pneumonia
Peripheral upper lung consolidation
Blood eosinophilia
Organizing Pneumonia
Idiopathic, collagen vascular diseases, and infections
Lower lobe and peripheral ground-glass opacity, small nodules, or focal consolidation
“Atoll” or “reverse halo” sign
Lipoid Pneumonia
Aspiration of oils used for laxatives
Lower lobe consolidation or mass
Central low-attenuation areas (-80 to -30 HU)
Langerhans Cell Histiocytosis
Centrilobular nodules ± central cavitation
Costophrenic angles spared
Round or bizarrely shaped cysts in upper lungs
Lymphangiomyomatosis
Women of childbearing age
Large lung volumes with chylous effusions and pneumothoraces
Numerous diffuse round lung cysts
Hypersensitivity Pneumonitis
Centrilobular nodules of ground-glass opacity
“Head-cheese” sign: Ground-glass opacity, decreased lung attenuation, and normal lung
Desquamative Interstitial Pneumonia
Diffuse/patchy ground-glass opacity
± cystic lesions or centrilobular emphysema
± lower lobe predominance
Image Gallery
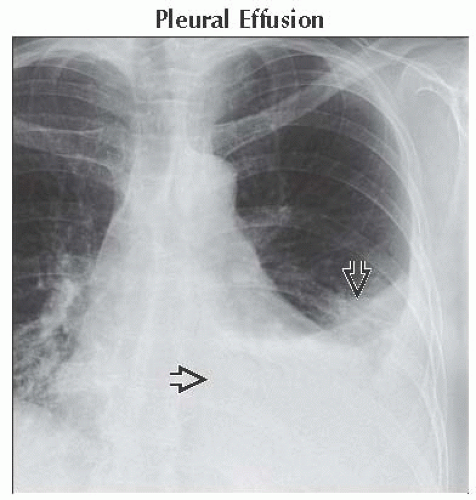
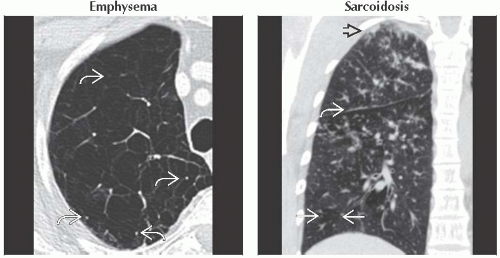 (Left) Axial CECT shows severe centrilobular emphysema with near complete destruction of the secondary pulmonary lobule. Note preservation of centrilobular core structures
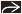 and lack of definable walls. (Right) Coronal NECT shows small perilymphatic lung nodules typically seen in sarcoidosis. Note the beaded major fissure and lack of definable walls. (Right) Coronal NECT shows small perilymphatic lung nodules typically seen in sarcoidosis. Note the beaded major fissure 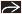 , subpleural nodularity , subpleural nodularity  , and lobular mosaic perfusion , and lobular mosaic perfusion  secondary to sarcoid granulomas involving small airways. secondary to sarcoid granulomas involving small airways.Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|