Chapter 7 Chemical Ablation
Historical Background
Historical data suggest that chemical ablation of the great saphenous vein (GSV) using liquid sclerosant delivered percutaneously via syringe and needle will fail (recanalize) in over 50% of cases at 1 year. Sclerotherapy in Europe became less frequently performed in the latter part of the 20th century, at least in part, because of the work of Hobbs.1 His 10-year randomized controlled study showed the clinical recurrence of varices was common in patients with truncal saphenous reflux managed with sclerotherapy.2 Hobbs found that after 10 years, 71% of patients treated with traditional surgery for saphenous incompetence had a good outcome; this compared with only 6% of patients treated by sclerotherapy. Recent scientific evidence has shown that liquid sclerotherapy is not very effective at eliminating truncal saphenous incompetence and failure to eliminate reflux leads to early recurrence of varices. Scientific evidence is limited when comparing sclerotherapy and surgery due to the fact that many of the trials had methodologic flaws such as lack of evaluation by independent observers, high dropout rates, poorly defined outcomes, and the lack of intention-to-treat analyses.3,4 However, despite these defects, most of the studies clearly demonstrated very high recurrence rates of varicose veins after sclerotherapy, ranging from 20% to 70%.
Etiology and Natural History of Disease
The efficacy of sclerosing agents is a function of concentration and vein diameter.5 If the target vein diameter is greater than 3 mm, liquid sclerosants do not properly reach the vein wall secondary to dilution. Sclerosant in the form of foam has clearly improved the results of sclerotherapy. Foam is more efficacious than liquid6–8 and is more readily monitored with ultrasound imaging. Foam is the reason chemical ablation has made a resurgence. Foam will expand and fill a vein of less than 12-mm diameter, offering better contact with the vein wall. Cabrera and colleagues9 published a clinical series of 500 lower limbs treated with foam sclerotherapy and reported that after 3 or more years, 81% of treated great saphenous trunks remained occluded and 97% of superficial varices had disappeared. This required one session of sclerotherapy in 86% of patients, two sessions in 11% of patients, and three sessions in 3% of patients.
Sclerosants
When Elkins Sinn discontinued production of Sotradecol in the United States in 2000, a nationwide shortage ensued. Since no other manufacturer had FDA approval to produce STS, compounding pharmacies were the only source from which physicians could obtain this agent. The shortage of STS and the stopgap role of compounding pharmacies ended in November 2004 when the FDA granted approval to Bioniche Pharma USA, Inc. (Belleville, Ontario, Canada) to manufacture STS in 1% and 3% strengths. Today, FDA-approved Sotradecol is manufactured by Bioniche Pharma in an FDA-approved facility and sold exclusively by AngioDynamics, Inc. (Queensbury, NY). In a study of compounded STS versus pharmaceutical-grade STS, several findings were reported.10 Compounded STS was found to contain measured levels of impurities, the most important of which was carbitol. Analysis of pharmaceutical-grade STS revealed no detectable levels of impurities. Although the level of STS impurity necessary to precipitate a clinical event is unknown, impurities in other drugs have been linked to significant unexpected adverse events. Concentrations of different compounded STS formulations showed significant variation when measured by an independent laboratory. In one sample, the concentration was 20% below the desired 3% concentration level.
Patient Selection
Sclerotherapy is a good choice for the treatment of nonsaphenous varicose veins, residual veins after surgical correction of axial vein reflux, and recurrent varicose veins secondary to neovascularization or incompetent perforating veins. Sclerotherapy is also the treatment of choice in spider telangiectasias, venectasias, and isolated reticular veins.11 The mode of action is induction of irritation of the vein wall followed by inflammation and fibrosis. Different agents are used such as hypertonic glucose that act by dehydrating the endothelium and substances like ethanolamine oleate8 that have a detergent effect in the endothelial layer.
Operative Steps
The three different methods of injecting large veins are:
Imaging
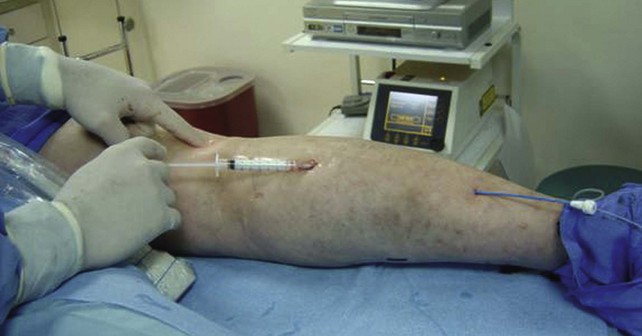
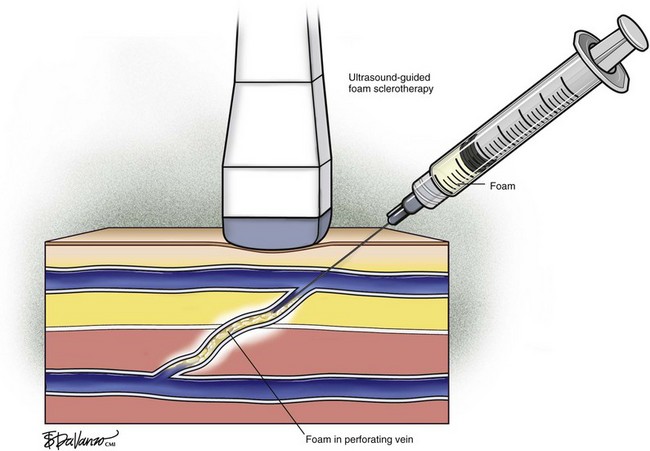
Recent trends have demonstrated a growing use of duplex-guided sclerotherapy, which was first described in 1989.15 It not only minimizes the chances of intraarterial injection and extravasation but also allows the estimation of the degree of spasm, length of vein treated, and position of the deep veins (Figs. 7-6 and 7-7).
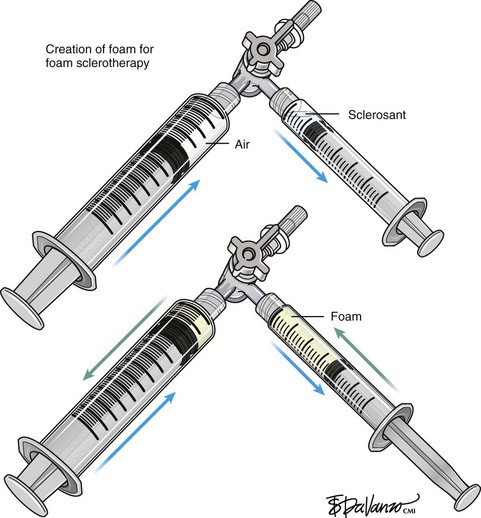
Foam Sclerotherapy
A recent revolution in the treatment of venous disease has been the emergence of foam sclerotherapy. Egmont James Orbach in 1944 first proposed the use of foam generated by a simple process of shaking a sclerosant with air. However, interest faded because it could only be used for small veins owing to large bubbles and a high air-to-liquid ratio.16 The renaissance of foam sclerotherapy is credited largely to the work of Cabrera et al.17 and Monfreux et al.18 in the 1990s.
Foam for the purpose of vein wall destruction is a nonequilibrated dispersion of gas bubbles in a sclerosing solution in which the gas fraction is 0.5221 or greater. The foam is composed of tiny bubbles of gas covered by a tensioactive liquid.19 Small bubbles make the foam highly interactive, whereas large bubbles produce ineffective foam. The foam mechanically displaces the blood and comes into contact with the endothelium. Therefore, the same concentration of the sclerosant is suitable for large and small veins. There is sufficient clinical evidence to demonstrate several advantages of foam preparations over conventional liquid sclerotherapy6,20:
Variables like type and concentration of the sclerosing agent, gas, gas-to-liquid ratio, bubble size, and time between preparation and use determine the efficacy of the agent.21 An ideal foam should be durable enough to allow injection before separating into gas and liquid components.22 It is accepted that microfoams with bubble diameters less than 250 µm are commonly used and the ideal effective choice (macrofoams have bubbles larger than 500 µm and minifoams have bubbles ranging from 250 to 500 µm). There are various techniques in the literature that describe effective ways to produce microfoam.
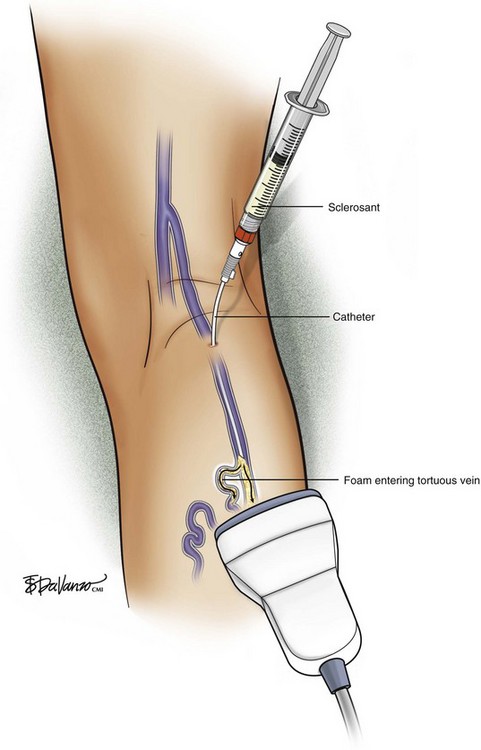
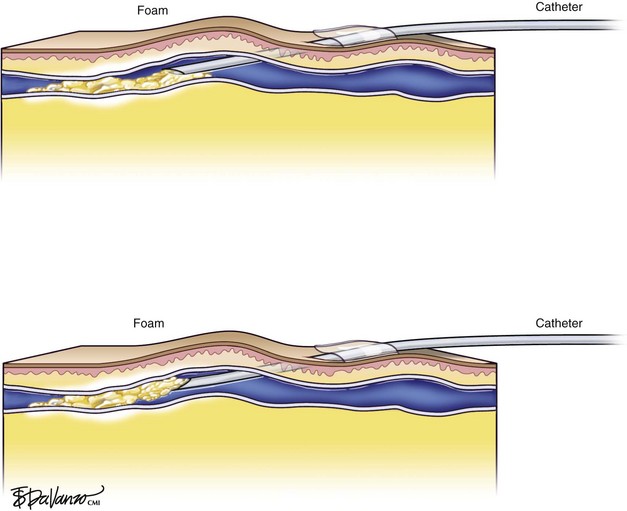
In reference to the biologic behavior of STS, Schneider and Fischer23 showed that endothelial damage is concentration dependent and occurs immediately after injection, with resulting rapid thrombus formation leading to vascular sclerosis. Importantly, 3% STS foam has not yielded 100% GSV closure when injected with a standard needle and syringe; therefore, catheters that can enhance the interaction of drug to vein wall are under investigation.
Foam is an option for controlling saphenous reflux in veins of less than 12 mm and has been shown to be a viable treatment for sclerosis of perforators. Foam is of value in the treatment of varicosed tributaries, tortuous vessels, and venous malformations. Foam also has limitations and carries liability concerns. In veins larger than 12 mm, the sclerosant–blood interface compromises treatment.24 It has been recommended that a 10-mL limit be placed with regard to total foam volume injected because of possible paradoxical embolization via the patent foramen ovale.19 For this reason there has been some interest in maximizing sclerosant contact time with the vein wall through the use of catheters. Future studies will likely focus on maximizing results of liquid or foamed sclerosants through innovations in catheter technology. The known methods of producing foam sclerotherapy include the following:
Cabrera method: Juan Cabrera in 1997 published his 7-year experience of excellent results with special foam that is prepared with a sclerosing agent, CO2, and an unknown tensioactive agent.17
Monfreux method: In this method, foam is produced in a glass syringe with the tip closed by a sterile plug; tension is applied by compressing the piston. While the foam is long lasting, the larger bubbles formed by this method can reduce the effectiveness of the therapy.18
Tessari method: Described in 1999 by Lorenzo Tessari, high-quality foam is produced with two disposable syringes and a three-way tap.24 STS was initially described with this method. The advantages of this method are use of disposable materials, compact foam with small bubble diameter, and the ability to reconstitute the foam if the treatment session takes more time.
Frullini method: Described by Frullini and Cavezzi in 2000, the foam generation uses the same turbulence effect as the Tessari method.18 Foam is generated by a vial and syringe joined by a connector.
Comparative Effectiveness of Existing Treatments
Results of Clinical Trials
A prospective randomized control trial involving more than 800 patients over 10 years compared six treatment options: liquid sclerotherapy, high-dose liquid sclerotherapy, multiple ligations, stab avulsions, foam sclerotherapy, and ligation followed by sclerotherapy. The conclusion was that the results of foam sclerotherapy were superior to those of liquid sclerotherapy and comparable with those of surgery.25
The listed complications ranged from phlebitis, skin necrosis, transient visual disturbance, and, on rare occasions, deep venous thrombosis (DVT). There have been suggestions that foam sclerotherapy may have a slightly higher rate of pigmentation, inflammation, and minimal necrosis when used in small reticular veins and telangiectasias.18 There have been uncertainties regarding the safety of foam preparations as animal studies demonstrated increased pulmonary hypertension. The stress on the human respiratory system is still unclear. The volume used per session depends on the size of the veins. No cases of DVT were reported in a prospective study of large volume foam sclerotherapy for varicose veins.26
Two randomized controlled trials (RCTs) compared foam sclerotherapy with high ligation and stripping (HL/S). The first RCT showed that HL/S is superior to foam sclerotherapy using Varisolve (BTG plc, London, UK) for occlusion and elimination of reflux (86% versus 63%) but that foam sclerotherapy was superior to conventional sclerotherapy (90% versus 76%).26 The second RCT showed that foam sclerotherapy combined with high ligation was less expensive, involved shorter treatment time, and resulted in more rapid recovery compared with HL/S.27
Although foam sclerotherapy is used universally and has become the standard of care, foamed sclerosants can embolize to the arterial circulation via patent foramen ovale. Only three permanent adverse events from paradoxical embolization of foam have been published28,29; however, several transient neurologic events have been reported anecdotally, and therefore foam remains controversial.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree