The optimal timing of coronary angiography in patients with non–ST elevation (NSTE) acute coronary syndromes (ACS) is debated. American Heart Association and American College of Cardiology guidelines recommend an early invasive strategy <12 to 48 hours after the onset of symptoms. The objective of the present study was to determine possible changes in myocardial function in patients with NSTE ACS awaiting coronary angiography. One hundred two patients with suspected NSTE ACS were enrolled, including 56 with NSTE myocardial infarctions (NSTEMIs), 23 with unstable angina pectoris, and 23 with noncoronary chest pain. Global and regional myocardial function was measured as longitudinal and circumferential strain using speckle-tracking echocardiography. Measurements were performed at admission and immediately before coronary angiography (30 ± 16 hours after admission). In patients with NSTEMIs, there was deterioration in longitudinal global strain from −16.1 ± 2.6% at admission to −15.0 ± 2.6% before coronary angiography (p <0.001). This was due to deterioration in longitudinal strain in the territory supplied by the infarct-related artery from −14.2 ± 4.2% to −12.0 ± 4.1% (p <0.001). Patients with NSTEMIs due to acute coronary occlusion underwent prominent worsening in longitudinal and circumferential strains (−15.7 ± 2.9% to −13.9 ± 3.0%, p = 0.001, and −16.7 ± 4.0% to −15.0 ± 3.9%, p = 0.01, respectively) compared to patients with NSTEMIs without occlusions. There were no changes in strain in patients with unstable angina pectoris or noncoronary chest pain. In patients with NSTEMIs without acute coronary occlusions, myocardial function improved after revascularization, whereas patients with acute occlusions demonstrated no improvement. In conclusion, myocardial function deteriorates in patients with NSTEMIs awaiting coronary angiography. Patients with acute coronary occlusions have the most prominent deterioration, and this subgroup shows no recovery of function after revascularization.
Non–ST elevation (NSTE) acute coronary syndromes (ACS), which comprise NSTE myocardial infarction (NSTEMI) and unstable angina pectoris, are more frequent than myocardial infarctions with ST elevations. The American Heart Association and American College of Cardiology guidelines recommend coronary angiography within 12 to 48 hours for risk stratification and planning of revascularization in patients with increased risk, whereas the European Society of Cardiology recommends angiography within 72 hours for high- and intermediate-risk patients. However, the optimal timing of coronary angiography for patients scheduled to receive this early invasive strategy remains unsolved. The NSTE ACS population is very heterogenous, with pathology ranging from discrete atherosclerosis in small-caliber coronary arteries to extensive disease with critical stenoses or occlusions of major vessels. Some patients may have ongoing myocardial ischemia until revascularization, and little is known about how this affects myocardial function. New echocardiographic modalities enable the accurate evaluation of global and regional myocardial deformation by strain and can identify abnormal myocardial function due to ischemia and necrosis. Echocardiography is easily accessible and can be performed repeatedly in the emergency or intensive care room. Consequently, strain by echocardiography is a feasible method for evaluation of myocardial function in patients with NSTE ACS to study changes in function due to ongoing ischemia. The aim of this study was to determine possible changes in myocardial function in terms of strain using speckle-tracking echocardiography in patients with NSTE ACS awaiting coronary angiography.
Methods
Patients with suspected NSTE ACS admitted to a local hospital were consecutively evaluated for study inclusion. Eligible patients had to fulfill 3 criteria: (1) acute anginal pain lasting ≥10 minutes and clinically classified as unstable angina pectoris or NSTEMI, (2) a history of chest pain <3 days, and (3) indication for coronary angiography according to current guidelines. Exclusion criteria were (1) age <18 years, (2) previous coronary artery bypass graft or valve surgery, (3) bundle branch block with QRS interval >0.12 seconds, (4) severe valvular dysfunction, (5) atrial fibrillation with heart rate >100 beats/min or continuous severe arrhythmia, and (6) severe mental disorder or short life expectancy because of extracardiac reasons. Because the aim of this study was to assess myocardial function in patients with NSTE ACS awaiting coronary angiography, patients who were planned for very early (<10 hours) angiography were not included. All patients received medical treatment according to guidelines. One hundred two patients were included and retrospectively grouped according to discharge diagnosis. NSTEMI and unstable angina pectoris were diagnosed by convention, using a cut-off value of troponin T >0.03 μg/L for NSTEMI. Patients without evidence of significant coronary artery disease were classified as having noncoronary chest pain (NCCP). The research protocol was approved by the regional committee for medical research and ethics. All participants gave written informed consent.
Echocardiographic examinations were performed using a Vivid 7 scanner (GE Vingmed Ultrasound AS, Horten, Norway). All recordings were digitally stored. Measurements were performed at admission and immediately before coronary angiography. Additionally, 95 patients (93%) were examined using echocardiography 99 ± 20 days after admission. Three consecutive heart cycles from 3 apical imaging planes (4 chamber, 2 chamber, and long axis) and 3 short-axis planes (mitral valve, papillary muscle, and apex) were obtained using 2-dimensional grayscale echocardiography. The mean frame rate was 74 ± 9 frames/s. Mitral inflow velocity was recorded by pulsed Doppler with the sample volume placed between the leaflet tips. Mitral annular velocities were recorded by tissue Doppler using color mode. Early diastolic mitral annular velocity (e′) was calculated by averaging septal and lateral annular velocities.
Echocardiographic recordings were analyzed by a single observer blinded to patient data, using EchoPAC version 7 (GE Vingmed Ultrasound AS). Longitudinal strain was computed on the basis of the apical imaging planes and circumferential strain on the basis of the short-axis planes. A 16-segment model of the left ventricle was obtained, and longitudinal and circumferential global strain was calculated by averaging all segmental peak systolic longitudinal and circumferential strain values. Longitudinal and circumferential territorial strain was calculated on the basis of the perfusion areas of the 3 major coronary arteries, as proposed by Cerqueira et al, by averaging all segmental peak systolic longitudinal and circumferential strain values within each territory. Segments not belonging to the culprit territory were averaged and defined as the remote area.
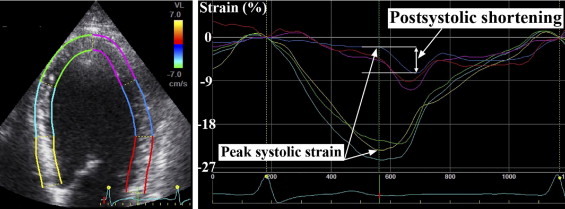
Normal segmental strain was defined as the average segmental strain in 17 patients without a history of heart disease, discharge diagnoses of NCCP, and no other serious conditions with possible influence on myocardial contractility. We defined hypercontraction as absolute segmental strain >1 SD from the normal segmental strain value. The percentage of segments within the remote area with evidence of hypercontraction was calculated for each patient. The influence of single- versus multivessel disease was studied in patients without a history of myocardial infarctions. Longitudinal postsystolic shortening was calculated on a segmental basis as the difference between longitudinal peak postsystolic strain and longitudinal end-systolic strain ( Figure 1 ). Longitudinal global postsystolic shortening was obtained by averaging all segmental postsystolic shortening values. Peak systolic strain was defined as the maximum absolute value of peak positive or peak negative strain during systole. End-systole was defined by aortic valve closure in the apical long-axis view. We analyzed 97.2% of the longitudinal and 92.0% of the circumferential segments. The left ventricular ejection fraction and end-diastolic volume were assessed using Simpson’s biplane method. The ratio of peak early mitral inflow velocity (E) divided by e′ was used as a noninvasive estimate of left ventricular diastolic filling pressure. Coronary angiograms were interpreted by experienced operators. The culprit lesion was described on the basis of the association between angiographic lesion morphology and electrocardiographic changes.
The data were analyzed using standard statistical software (SPSS version 16.0; SPSS, Inc., Chicago, Illinois). Values are presented as number (percentage) or as mean ± SD. Differences between groups were analyzed using 1-way analysis of variance for continuous variables, and Bonferroni’s correction was applied for post hoc tests. For categorical variables, differences between groups were analyzed using chi-square or Fisher’s exact tests. Assessments of changes in strain within groups were performed using paired Student’s t tests. Differences between groups regarding change in strain, echocardiographic, and clinical parameters were tested with general linear models, and Bonferroni’s correction for multiple comparisons was applied. Relations between the levels of biomarkers and the level of strain were tested using linear regression. Reproducibility was calculated by intraclass correlation in 10 randomly selected patients. For all analyses, p values <0.05 were considered significant.
Results
Baseline characteristics are listed in Table 1 . Clinical and conventional echocardiographic parameters at admission and before coronary angiography are given in Table 2 . There were no differences between the 3 groups in time from symptom onset to admission or from admission to coronary angiography. Furthermore, there were no differences between patients with NSTEMIs with and without acute coronary occlusions with regard to these time intervals (time to admission 12 ± 14 vs 8 ± 8 hours, time from admission to coronary angiography 29 ± 15 vs 34 ± 18 hours).
| Variable | NSTEMI | UAP | NCCP |
|---|---|---|---|
| (n = 56) | (n = 23) | (n = 23) | |
| Age (years) | 67 ± 14 ⁎ | 58 ± 11 | 56 ± 10 |
| Men/women | 41:15 | 14:9 | 13:10 |
| Previous myocardial infarction | 7 (13%) | 7 (30%) | 2 (9%) |
| Previous PCI | 2 (4%) ⁎ | 7 (30%) | 6 (26%) |
| Time from symptom onset to admission (hours) | 9 ± 10 | 13 ± 15 | 13 ± 18 |
| Time from admission to angiography (hours) | 32 ± 17 | 32 ± 17 | 32 ± 17 |
| Medications before hospitalization | |||
| Acetylsalicylic acid | 18 (32%) | 10 (44%) | 7 (30%) |
| Clopidogrel | 3 (5%) | 4 (17%) | 2 (9%) |
| β blockers | 15 (27%) | 9 (40%) | 5 (22%) |
| ACE inhibitors/ARBs | 23 (41%) | 9 (39%) | 5 (22%) |
| Calcium inhibitors | 6 (11%) | 1 (4%) | 1 (4%) |
| Statins | 20 (36%) | 11 (48%) | 10 (44%) |
| Medications before coronary angiography | |||
| Acetylsalicylic acid | 56 (100%) | 23 (100%) | 23 (100%) |
| Clopidogrel | 56 (100%) | 22 (96%) | 23 (100%) |
| Low–molecular weight heparin | 55 (98%) | 22 (96%) | 22 (96%) |
| Glycoprotein IIb/IIIa inhibitors | 12 (21%) | 1 (4%) | 1 (4%) |
| β blockers | 49 (88%) | 19 (83%) | 16 (70%) |
| ACE inhibitors/ARBs | 27 (48%) | 11 (48%) | 6 (26%) |
| Calcium inhibitors | 7 (13%) | 1 (4%) | 1 (4%) |
| Nitroglycerin | 29 (52%) | 9 (39%) | 7 (30%) |
| Statins | 56 (100%) | 23 (100%) | 17 (74%) † |
| Variable | NSTEMI | UAP | NCCP | |||
|---|---|---|---|---|---|---|
| Admission | CA | Admission | CA | Admission | CA | |
| Systolic blood pressure (mm Hg) | 137 ± 25 | 134 ± 19 | 140 ± 25 | 133 ± 23 | 135 ± 17 | 129 ± 16 |
| Heart rate (beats/min) | 70 ± 12 | 70 ± 19 | 66 ± 10 | 66 ± 9 | 69 ± 11 | 64 ± 12 |
| Left ventricular ejection fraction (%) | 53 ± 6 | 51 ± 7 | 54 ± 8 | 54 ± 6 | 59 ± 7 ⁎ | 59 ± 5 |
| End-diastolic volume (ml) | 107 ± 30 | 106 ± 32 | 103 ± 29 | 110 ± 40 | 103 ± 24 | 106 ± 16 |
| E/e′ ratio | 10 ± 4 | 11 ± 4 † | 9 ± 2 | 9 ± 2 | 8 ± 3 | 8 ± 2 |
⁎ p <0.05 vs NSTEMI and UAP at admission;
The culprit lesions were identified and localized to major coronary vessels in 47 patients with NSTEMIs and in 15 with unstable angina pectoris. Lesions were equally distributed among the 3 major coronary territories (left anterior descending coronary artery n = 21, circumflex coronary artery n = 19, and right coronary artery n = 22). In the NSTEMI group, 16 patients (29%) had acute coronary occlusions, and 27 (48%) had ≥90% stenoses. Percutaneous coronary intervention was performed in 39 patients with NSTEMIs (70%) and in 9 patients with unstable angina pectoris (39%), whereas coronary artery bypass grafting was performed in 8 (14%) and 5 (22%) patients, respectively.
The average longitudinal and circumferential segmental strain in healthy subjects were −19.6 ± 4.2% and −21.9 ± 7.3%, respectively.
The main results of the study are listed in Tables 3 and 4 . At admission, longitudinal and circumferential strain were impaired in patients with NSTEMIs compared to those with NCCP. Longitudinal global strain deteriorated from admission to coronary angiography in patients with NSTEMIs. In patients with NSTEMIs and acute coronary occlusions, there was a pronounced deterioration of longitudinal and circumferential global strain from admission to coronary angiography, both significantly more prominent than in patients with nonocclusive disease ( Table 4 ). Additionally, peak troponin T and creatine kinase-MB in patients with acute occlusions were considerably higher than in patients with NSTEMIs without occlusions (troponin T 3.3 ± 2.5 vs 0.9 ± 1.3 μg/L, p <0.001; creatine kinase-MB 107 ± 78 vs 38 ± 40 μg/L, p <0.001). Peak troponin T and creatine kinase-MB were significantly related to strain in the culprit territory before coronary angiography (troponin T vs culprit territorial longitudinal strain R = 0.59, p <0.001; creatine kinase-MB vs culprit territorial longitudinal strain R = 0.52, p <0.001). No changes in circumferential global strain were observed in patients with NSTEMIs without acute coronary occlusions, in patients with unstable angina pectoris, or in those with NCCP. The extent of deterioration in longitudinal global strain in patients with NSTEMIs was not associated with which perfusion territory was affected (changes in longitudinal global strain: left anterior descending coronary artery 1.5 ± 1.8%, circumflex coronary artery 1.4 ± 1.4%, right coronary artery 1.2 ± 1.4%; p = 0.88). There were no differences in longitudinal or circumferential global strain with regard to which coronary territory was affected (p = 0.47 and p = 0.64, respectively).
| Variable | Admission | CA | ΔStrain | p Value ⁎ |
|---|---|---|---|---|
| NSTEMI (n = 56) | ||||
| Longitudinal strain (%) | −16.1 ± 2.6 † | −15.0 ± 2.6 † ‡ | 1.1 (0.7 to 1.5) † ‡ | <0.001 |
| Circumferential strain (%) | −18.4 ± 5.1 † | −18.2 ± 5.4 † | 0.2 (−0.6 to 1.1) | 0.53 |
| UAP (n = 23) | ||||
| Longitudinal strain (%) | −17.2 ± 3.0 † | −17.6 ± 2.4 | −0.4 (−1.0 to 0.2) | 0.20 |
| Circumferential strain (%) | −19.8 ± 4.6 | −19.3 ± 3.9 | 0.5 (−0.4 to 1.4) | 0.23 |
| NCCP (n = 23) | ||||
| Longitudinal strain (%) | −19.5 ± 2.0 | −19.3 ± 2.1 | 0.2 (−0.4 to 0.8) | 0.44 |
| Circumferential strain (%) | −22.0 ± 3.1 | −22.2 ± 3.0 | −0.2 (−1.1 to 0.7) | 0.63 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


