Cerebral and Peripheral Angiography
Inder M. Singh
Steven J. Filby
Mehdi H. Shishehbor
Noncoronary angiography, often generalized as “peripheral” angiography, has become an increasingly common part of the invasive cardiologist’s repertoire. Prior to gaining a sound working knowledge of peripheral angiography, it is important to recognize several associated salient technical features. It is also prudent to have a sound knowledge of the key elements that distinguish peripheral from coronary angiography.
The diameter of the image intensifier used for peripheral angiography is 14 to 16 in compared to 9 in used in most cardiac catheterization laboratories. This provides a larger imaging window when viewing the aorta and lower extremities.
The frame rate for peripheral angiography is usually 2 to 3 frames per second compared to 15 to 30 frames per second for coronary angiography.
Digital subtraction angiography (DSA) is the preferred imaging modality for peripheral angiography. DSA literally subtracts the surrounding radio structures (such as bone) and shows only the contrastfilled arterial bed of interest. This technique greatly enhances image quality but requires the patient to be completely still (including temporary cessation of respiration or swallowing for carotid angiography) during image acquisition. The radiation exposure for DSA is significantly greater than standard cardiac cineangiography.
Trace subtract fluoroscopy or “road mapping” is frequently used instead of standard fluoroscopy during peripheral angiography. Like DSA, “road mapping” also enables visualization of contrast-filled vessels without the surrounding tissues or bone.
Arterial access for peripheral angiography may require retrograde common femoral artery (CFA) cannulation. However, depending on the nature of disease, antegrade CFA cannulation or brachial/radial
access may be required. Prior to upper extremity access, an Allen test should be performed to confirm intact dual vascular supply of the hand. Familiarity with the micropuncture system is essential for all physicians performing peripheral angiography.
Ionic contrast is not used for peripheral angiography as it has been associated with transient vision loss during cerebrovascular angiography and is extremely painful when used for limb angiography. A nonionic iso-osmolar agent such as iodixanol is the preferred contrast agent.
Similarities in the periprocedural evaluation of patients undergoing coronary or peripheral angiography are enumerated below.
Knowledge of presenting symptoms is essential for clinical and technical decision making.
Knowledge of comorbid medical conditions is important for performing the procedure safely.
Knowledge of angiography and prior revascularization in any vascular bed is imperative. Reviewing noninvasive data from peripheral computed tomography and magnetic resonance angiography can be extremely helpful for planning access and strategy. This becomes especially important in cases of complete occlusion and also in cases of prior bypass grafting. It is also important to note the timing of bypass grafts, as direct cannulation of grafts less than 6 months old should be avoided when possible.
A focused physical examination pre- and post-procedure is mandatory and should include access site examination and a full assessment of all relevant vascular beds proximally and distally for pulses, bruit, thrills, or presence of hematoma. Additionally, a pre- and postfocused neurologic examination should be performed for carotid and cerebral angiography.
Standard preangiography laboratory evaluation is necessary for patient safety.
Medications such as warfarin and metformin should be held prior to peripheral angiography in a manner similar to coronary angiography.
Cerebrovascular
Anatomy:
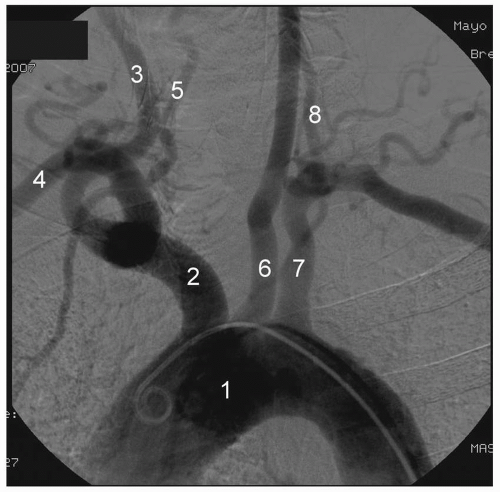
The aortic arch is the major conduit that gives rise to the entire cerebrovascular system (Figure 6-1). The aortic arch is classified as type 1, 2, or 3 based on plane and angle of takeoff of its major branches. As part of the aging process, the aortic arch can remodel from type 1 (horizontal plane) to type 3 (C-shaped plane) as the arch sinks into the thoracic cavity. Traditionally, the arterial branches arise off the arch in
the following order: (1) innominate artery, (2) left common carotid artery (CCA), and (3) left subclavian artery (SCA). The innominate bifurcates into the right SCA and the right CCA. The right and left SCA give rise to their respective vertebral arteries. A more detailed description of the SCA and its branches is presented in the “Upper Extremity/Thorax” section later in this chapter.
the following order: (1) innominate artery, (2) left common carotid artery (CCA), and (3) left subclavian artery (SCA). The innominate bifurcates into the right SCA and the right CCA. The right and left SCA give rise to their respective vertebral arteries. A more detailed description of the SCA and its branches is presented in the “Upper Extremity/Thorax” section later in this chapter.
The right CCA usually arises from the bifurcation of the innominate artery (Figure 6-1). Occasionally, the right CCA may arise independently from the aortic arch. Rarely, the right and left CCA may arise as a common carotid trunk from the arch. The left CCA has significant variation in its anatomy. In 75% of individuals, it is the second great vessel arising from the aortic arch, posterior to the innominate. In the remaining cases, the origin of the left CCA is via a shared origin with
the innominate off the aortic arch or as a branch off the innominate proper (also known as a “bovine arch”). Although there is significant variation, the CCA typically bifurcates into the external carotid artery (ECA) and the internal carotid artery (ICA), at the upper border of the thyroid cartilage (Figure 6-2). The CCA usually does not give off any significant branches until it bifurcates into the ICA and ECA.
the innominate off the aortic arch or as a branch off the innominate proper (also known as a “bovine arch”). Although there is significant variation, the CCA typically bifurcates into the external carotid artery (ECA) and the internal carotid artery (ICA), at the upper border of the thyroid cartilage (Figure 6-2). The CCA usually does not give off any significant branches until it bifurcates into the ICA and ECA.
The ECA is readily recognized due to its eight extracranial branches. These arterial branches arise in the following order—superior thyroid, ascending pharyngeal, lingual, occipital, facial, posterior auricular, maxillary, and superficial temporal (Figure 6-2).
The ICA is recognized by the bulbous carotid sinus at its origin. This region houses the mechano- and chrono-regulatory receptors for the body. Conventionally, the ICA is divided into four segments— cervical, petrosal, cavernous, and supraclinoid (sometimes also referred to as subarachnoid).
Cervical segment: The segment between the CCA bifurcation and the petrous bone. This segment does not give off any arterial branches. The ostial and proximal portions of this segment are often involved in carotid atherosclerotic disease.
Petrosal segment: The segment that courses through the petrous bone to the cavernous sinus. This segment has the shape of an inverted hockey stick. This segment also does not give off any arterial branches.
Cavernous segment: The segment that courses through the cavernous sinus giving off the meningiohypophyseal and the inferior cavernous sinus arterial branch.
Supraclinoid segment: The segment begins after the ICA exits the cavernous sinus. Several key branches that originate from this segment include the ophthalmic, posterior communicating, and anterior choroidal. The ICA terminates after this segment in the anterior and middle cerebral artery. Thus, the main area of the brain supplied by the anterior and middle cerebral arteries is referred to as the carotid territory. The classical finding of amaurosis fugax results from ophthalmic artery ischemia.
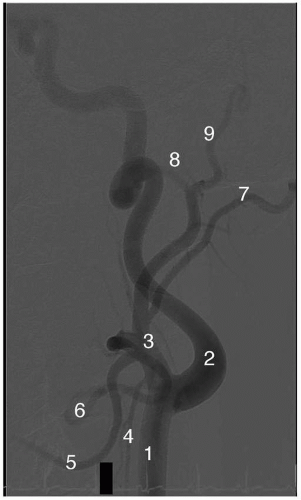
Other important extracranial vessels include the bilateral vertebral arteries that anastamose at the base of the pons to give rise to a single basilar artery, which lies intracranially. The largest branches of the vertebral arteries are the bilateral posterior inferior cerebellar arteries, while the basilar artery gives off bilateral anterior inferior cerebellar arteries, superior cerebellar arteries, and the posterior cerebral arteries. These arteries form the posterior circulation of the brain including the cerebellar blood supply. Usually, the left vertebral circulation is dominant and provides the majority of the arterial supply to this region.
The intracranial circulation is formed by the anterior cerebral artery, anterior communicating artery, middle cerebral artery, posterior cerebral artery, and posterior communicating artery (Figure 6-3). The circle of Willis is formed by these vessels but a “normal ring” is found only in 50% of individuals. Description of the anatomic variations is beyond the scope of this text.
Angiography:
Aortic arch angiography is the recommended first step prior to selective angiography of the cerebrovascular system (Figure 6-1). This allows for assessment of arch type, detection of anomalous vessel origin or takeoff, and estimation of proximal vessel atherosclerosis and tortuosity. Arch angiography is generally performed with a power contrast injection (20 mL per second for 40 mL total) using a standard multihole pigtail catheter approximately 40° left anterior oblique (LAO). For arch angiography, the patient should be asked to turn his or her head to the right in an extended position.
Carotid angiography and catheter selection is determined by aortic arch type. In type 1 and most type 2 arches, the carotids can be successfully engaged using a JR4 diagnostic catheter. For type 2 arches with bovine left CCA origin or type 3 arches, a Vitek catheter is usually required. A heparin bolus of 2,000 to 3,000 units should be administered prior to selective angiography of the cerebrovascular system. Before engaging the great vessels, the arch angiogram should be used as a reference view on the monitor. For the right CCA, the innominate is first engaged; and the right anterior oblique (RAO) projection is used to separate the ostia of the right-sided SCA and CCA. In most cases, the diagnostic catheter can be carefully advanced to engage the right CCA. If there is any difficulty in engaging the ostium of the right CCA, a soft wire such as a Wholey or a stiff-angled Glidewire can be used to advance the catheter to the proximal portion of the CCA. The left CCA is selectively engaged directly off the aortic arch. In general, all catheter advancements should be performed over a wire.
The standard views to assess for carotid disease are ipsilateral oblique views between 30° and 45° and the left lateral view to
initially define the carotid bifurcation. The angle of mandible serves as a useful landmark for carotid bifurcation. Since the proximal ICA lies posterior and medial to the ECA, lateral and posteroanterior (PA) views may also be necessary to define the anatomy (Figure 6-2).
initially define the carotid bifurcation. The angle of mandible serves as a useful landmark for carotid bifurcation. Since the proximal ICA lies posterior and medial to the ECA, lateral and posteroanterior (PA) views may also be necessary to define the anatomy (Figure 6-2).
Troubleshooting
Air and clot embolization: Embolization of either air or clots in the cerebral vasculature can lead to catastrophic consequences. Thus, a meticulous technique with regular checks for air or clots and frequent flushing of catheters with heparanized saline is essential. Catheter exchanges should be kept to the minimum during cerebrovascular angiography. When exchanges are performed, extreme care should be taken to wipe the wire of any clots and to flush catheter and access sheath.
Ostial stenosis of the vertebral artery: When the vertebral artery ostium is diseased, direct engagement with a diagnostic catheter is not recommended as this may cause embolization of the ostial plaque and posterior territory infarct. Thus, in situations of verterbral ostial disease, a nonselective angiogram should be obtained instead as described above.
Selective engagement of the vertebrals can often be achieved using a JR4 catheter or a Headhunter catheter using the same technique as with the carotids. However, nonselective angiography of the vertebrals is routinely performed with the tip of the diagnostic catheter close to but not directly engaged with the ostia. An ipsilateral arm blood pressure cuff is inflated to maximize visualization during nonselective angiograms. A single ipsilateral oblique view is adequate in most cases.
The anterior and middle cerebral circulation is best visualized in a shallow PA view at 15° to 30° (Figure 6-3). Positioning the petrous bone at the base of the orbit in this projection serves as a useful landmark. The intracranial posterior circulation is best seen in a steep PA view at 40° (Figure 6-3). Lateral views are also frequently used for all three intracranial circulations.
Upper Extremity
Anatomy:
The right SCA arises from the bifurcation of the innominate, whereas the left SCA arises as the third and final branch off the aortic arch (Figures 6-1 and 6-4). In 0.5% percent of cases, the right SCA arises as the last branch of the descending thoracic aorta. In its proximal segment, the SCA first gives off the vertebral artery followed by the internal mammary artery (IMA), the latter of which supplies the anterior chest wall (Figure 6-4). In 1% to 5% of individuals, the left vertebral artery arises directly from aortic arch. The thyrocervical and costocervical trunks arise from the midsegment of SCA and give
branches to the thyroid gland, cervical muscles, ribs, and the scapular region (Figure 6-4).
branches to the thyroid gland, cervical muscles, ribs, and the scapular region (Figure 6-4).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree