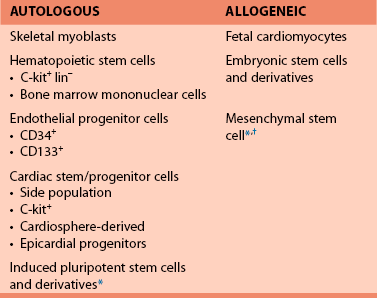
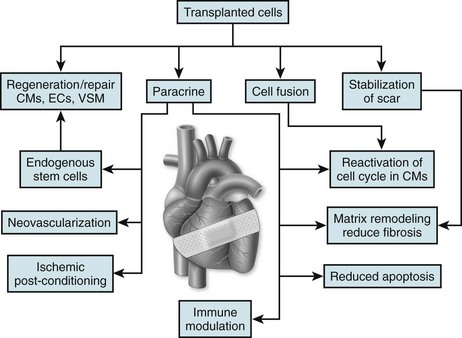
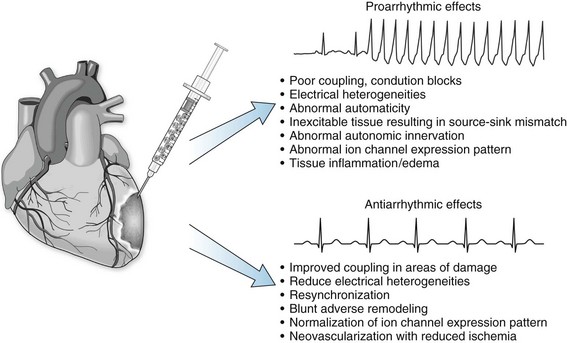
57 In the past decade, insights regarding the regenerative capabilities of the heart have offered new hope for the treatment of heart disease. Although the adult human heart has been described as a postmitotic, terminally differentiated organ, new studies have provided evidence that the heart is a dynamic organ with turnover of cells throughout life, including cardiomyocytes.1,2 Like many other organ systems, tissue-specific stem and progenitor cells have been identified recently; they provide a source for generating new cardiomyocytes and other essential cell types in the heart.3–5 However, endogenous cardiac stem cells are unable to generate adequate numbers of cardiomyocytes to repair the heart after large insults, such as myocardial infarction. Furthermore, the capacity of intrinsic repair by cardiac stem cells declines with age.6 Thus, the concept of delivering new, viable cells to the heart for repair and regeneration has been investigated aggressively over the past decade for treatment after myocardial infarction and in heart failure. Ideally, such cell-based therapy will lead to regenerated myocardium that exhibits normal functional properties and consequently reduces the risk of arrhythmias as the abnormal substrate is replaced, and the conditions that trigger arrhythmias are eliminated. However, the delivery of cells to the myocardium can also potentially introduce conditions that increase the risk for arrhythmias. The purpose of this chapter is to examine the electrophysiological consequences of cell therapy for heart disease based on existing experimental data and early clinical experience. A wide variety of different cell sources have been investigated for their ability to repair the heart in animal models and some in clinical trials. Advances in stem cell research over the past two decades have also contributed to the variety of cells types under consideration. Cells from the recipient of the graft (autologous) and isolated from donors (allogeneic) have been tested (Box 57-1). Autologous cell sources hold the advantage of not being recognized by the immune system as foreign, but they have the disadvantage of potentially costly individualized cell processing and the possibility that the disease phenotype will make the transplanted cells dysfunctional. Alternatively, allogeneic cells may be at risk for immune rejection, but the ability to optimize and manufacture large batches of quality-controlled cells for use in multiple patients could be advantageous. The cell sources vary dramatically in their properties, including proliferative capacity, potency, ability to differentiate into different cell types, ability to survive ischemic and inflammatory insults, and secretion of signaling molecules. Furthermore, it is likely that the different cell sources can differentially affect the electrophysiological properties either acutely or over time. Initial investigations in cell therapy for the heart sought the goal of remuscularizing the tissue by providing differentiated myocytes. The first cell source studied in detail was skeletal myoblasts derived from skeletal muscle satellite cells.7,8 Satellite cells can be isolated from a muscle biopsy and differentiated into myoblasts that can be expanded greatly in culture. Transplanted myoblasts formed viable grafts in animal hearts and improved functional properties of the hearts9,10; however, the transplanted cells formed skeletal muscle grafts, not cardiac muscle. This result had consequences because skeletal muscle lacks connexin expression and gap junctions; therefore, the cells did not electrically integrate into the myocardium.10,11 Alternatively, transplanted fetal mouse ventricular myocytes were demonstrated to integrate into a recipient mouse heart forming intercalated discs between donor cells and the recipient myocardium.12 Treatment of infarcted or cryoinjured myocardium with fetal cardiomyocytes in several animal studies resulted in improved left ventricular function and limited adverse myocardial remodeling compared with sham control animals.13,14 However, fetal cardiomyocytes are poorly tolerant of acute ischemia, with the vast majority not surviving the transplant. Fetal cardiomyocytes are also terminally differentiated, and they do not exhibit significant cell division at the site of engraftment. Therefore, it is difficult to obtain adequate cell numbers for larger areas of damage. Finally, the major limitation of applying this strategy to clinical medicine is the ethical objection to the use of human fetal tissue as well as the limited supply of such tissue. In 2001, Orlic et al.15 demonstrated that bone marrow–derived lineage-negative (lin−) and c-kit+ stem cells injected after myocardial infarction in a mouse model resulted in remarkable repair of the heart accompanied by functional improvement.15 The appealing concept that delivering stem cells to the injured heart could lead to robust regeneration of functional myocardium was put forward, but soon these results were challenged by others arguing against the ability of a hematopoietic stem cell to form cardiac tissue.16,17 Nevertheless, investigators considered other cells sources, such as mesenchymal stem cells (MSCs) derived from bone marrow to treat the injured heart, likewise suggesting the generation of new myocardium; however, this conclusion has also been challenged.18,19 The relatively recent demonstration of rare endogenous cardiac stem and progenitor cells in the heart provides additional possible cell sources for cardiac repair. Investigators have used a number of different techniques and cell surface markers to isolate endogenous cardiac progenitors. In the earliest study, progenitors were suggested based on the ability to expel Hoechst 33342 dye which was used with fluorescence-activated cell sorting (FACS) to identify a rare “side population” of cells in a similar fashion as had previously been done in hematopoietic cells.20 The side population cells have cardiac potential based on in vitro studies. The cell surface marker c-kit was subsequently used to identify multipotent cardiac stem cells in mouse and human hearts.21, 22 Likewise Sca-1 was identified as a cell surface marker for cardiac progenitors, but this protein is not expressed in the humans.4 The transcription factor Isl-1 has also been used to define a cardiac progenitor cell population in transgenic mouse models and in the human heart.5, 23 Explanted cardiac tissue can be cultured under conditions promoting the migration of cells that can be isolated and cultured and expanded under nonadherent conditions to form cardiospheres.24,25 These cardiospheres contain a mixed population of progenitors with the ability to form multiple lineages including cardiomyocytes. Finally, the epicardium has been reported to contain multipotent epicardial progenitors identified by WT-1 or Tbx-18, which can give rise to multiple cell types in the myocardium.26,27 The differences between the various progenitor populations is still the subject of intense investigation, and it is possible that different strategies identify some related populations at different stages of maturation.28 Nevertheless, transplanting such progenitor cells holds appeal for cardiac repair, and initial animal studies have shown functional benefit after myocardial infarction.3, 15, 29 The establishment of technologies to produce human pluripotent stem cells created additional considerations for cardiac cell therapy. The successful isolation of human embryonic stem cells (ESCs) from surplus in vitro fertilization embryos by Thomson et al.30 created new avenues for cardiac therapeutic applications.30 Subsequent demonstration that human ESCs cells could differentiate into functional cardiomyocytes confirmed the potential utility of this cell source for cardiac repair.31,32 Studies in animal models with mouse ESCs showed that the cells could have beneficial effects after myocardial infarction and generate different cell lineages following transplantation of undifferentiated ESCs.33–35 However, transplanting the ESCs without differentiation carries the risk of teratoma tumor formation36; therefore, subsequent studies have evaluated differentiated derivatives for repair.37,38 Even more recently, the remarkable reprogramming of somatic cells, such as dermal fibroblasts, to induced pluripotent stem cells has provided a pluripotent stem cell source that can potentially be genetically identical to the patient.39,40 Initial studies using this cell source have shown early promise,41 but many questions remain regarding the stability of the phenotype and the long-term effects of reprogramming. As investigators more critically examined cell survival and engraftment after cell delivery, it became progressively clear that many of the functional benefits observed in animal models were likely not due to simple remuscularization. The majority of transplanted cells, regardless of source, did not survive let alone generate new myocardium. Nevertheless, clear beneficial effects of the therapies were observed based on the functional and structural properties of treated hearts, and a number of additional mechanisms of benefit have been proposed (Figure 57-1). Perhaps most prominent among the potential mechanisms of benefit is the reported paracrine effects of certain cell populations, such as MSCs, to secrete molecules to promote survival of existing heart tissue and blunt adverse remodeling.19,42 Other potential beneficial effects include activating endogenous stem cells, cell fusion, induction of angiogenesis, antiinflammatory effects, and resynchronizing the myocardium. The exact mechanistic effect likely varies with different cell sources, and these details are far from completely defined in animal studies. Nevertheless, these early animal studies have generated sufficient interest and data to proceed quickly to clinical trials. Cellular grafts need to undergo electrical and mechanical integration into the myocardium for optimal benefit. Furthermore, the functional properties of the cells ideally must match those of normal myocardium. To investigate and optimize these features of cell therapies, studies have been performed using in vitro and animal models. Depending on the precise details of the grafts and their integration, the transplanted cells can be either proarrhythmic or antiarrhythmic (Figure 57-2). Cell therapy can contribute to the genesis of arrhythmias by affecting all three basic mechanisms of arrhythmias: reentry, abnormal automaticity, and triggered activity. Alternatively, cell therapy can blunt arrhythmias by improving the underlying substrate and removing triggers. Careful examination of the integration and functional properties of the cellular grafts is essential for optimizing safe and effective cell therapy approaches. As an initial test of the ability of donor cells to couple with native cardiomyocytes, a number of in vitro coculture experiments have been performed. These cocultures of donor cells with ventricular cardiomyocytes have highlighted different forms of electromechanical coupling. In the case of cocultured human ESC-derived cardiomyocytes, clear coupling with rat neonatal ventricular myocytes has been demonstrated with the formation of connexin43 (Cx43) gap junctions.43 Synchronized contractions were observed in the cocultures, suggesting clear functional coupling. In studies coculturing MSCs with rat ventricular myocytes, formation of Cx43 gap junctions was also observed, but in this case electrotonic conduction occurred via MSCs because these cells are not electrically excitable.44,45 In contrast, coculture of skeletal myoblasts with neonatal rat ventricular cardiomyocytes failed to couple because myotubes do not express Cx43 or form gap junctions.46 Thus, islands of electrically isolated myotubes provided substrate for reentry involving the surrounding cardiomyocytes. However, genetically engineered expression of Cx43 in myoblasts can lead to functional coupling with neonatal cardiomyocytes.46 These simple coculture studies highlight key differences in coupling that can occur after cell therapy to the myocardium, but understanding the full complexity of electromechanical coupling resulting from cell therapy requires study in intact hearts. A wide range of different cell sources have been tested in various animal models of cardiac injury; however, only a small minority of the studies has rigorously investigated the electrophysiologic consequences of cell therapy. Transplanted skeletal myoblasts were first examined for their ability to couple to native myocardium. Despite being transplanted into the heart, myoblasts differentiate into skeletal myotubes lacking Cx43 and do not couple to the post–myocardial infarction rat heart,47,48
Cell Therapy and Regenerative Electrophysiology
Introduction to Cardioregenerative Medicine
Cell Sources for Cardiac Repair
Basic Mechanisms by Which Cell Therapy Can Affect Cardiac Electrophysiology
Cell Coupling and Integration
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Cell Therapy and Regenerative Electrophysiology