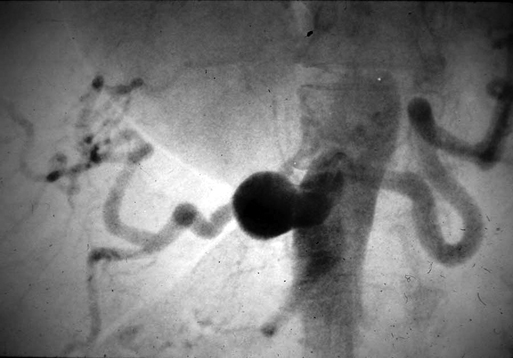
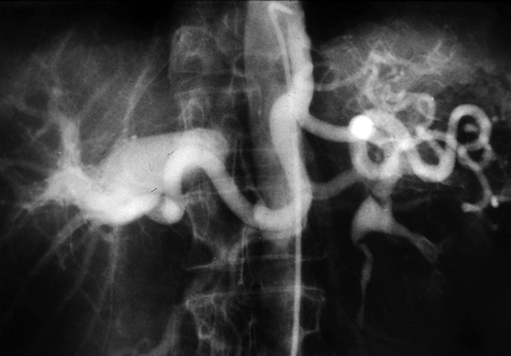
Currently, most celiac artery aneurysms are asymptomatic or are associated with vague abdominal discomfort. They are often first recognized as incidental findings on computed tomography or other imaging for nonvascular diseases (Figure 1). Some patients, however, have symptoms, with abdominal or back pain being the most common manifestation in these cases. Less common presentations are early satiety, jaundice, and gastrointestinal bleeding. Rupture reportedly occurs in 13% of these celiac artery aneurysms, is usually intraperitoneal, and carries a mortality of 50%. Intervention is warranted for all symptomatic celiac artery aneurysms and for bland aneurysms exceeding 2 cm in diameter. Most hepatic artery aneurysms are asymptomatic and are discovered incidentally during imaging for other illnesses (Figure 2). Those aneurysms that are symptomatic produce right upper quadrant and epigastric pain in half the patients. Acute expansion of hepatic artery aneurysms can cause severe abdominal discomfort, similar to that of acute pancreatitis. Large aneurysms have been reported to be a rare cause of obstructive jaundice.
Celiac, Hepatic, and Splenic Artery Aneurysms
Celiac Artery Aneurysms
Clinical Presentation
Hepatic Artery Aneurysms
Clinical Presentation
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Thoracic Key
Fastest Thoracic Insight Engine