CATHETER INTERVENTIONS IN THE NEONATE:
PART II—BALLOON ANGIOPLASTY/VALVULOPLASTY
Introduction
Several transcatheter interventions (Table 20.1) are useful in the management of the neonate with heart disease. In the preceding chapter, nonsurgical atrial septostomy was reviewed. Atrial septostomy was the major, if not the only, catheter interventional procedure available for treatment of children with heart disease until late 1970s. During the next decade, the technique of balloon angioplasty1 described by Gruntzig was adopted to treat pulmonary valve (PV) stenosis,2 native aortic coarctation,3 postsurgical aortic recoarctation,4 aortic valve stenosis,5,6 mitral valve (MV) stenosis,7 subaortic membrane,8 branch pulmonary artery (PA) stenosis,9 pulmonary vein stenosis,10 stenotic bioprosthetic valves,11,12 and other obstructive vascular lesions,13–18 These techniques were then applied to neonates with heart disease.4,19–22 In this chapter, balloon angioplasty/valvuloplasty for critical obstructive lesions of the heart in the neonate will be discussed.
Table 20.1. Catheter interventional techniques used in the neonate
| Nonsurgical atrial septostomy |
| Balloon angioplasty/valvuloplasty |
| Radiofrequency perforation of atretic PV |
| Transcatheter occlusion of shunts |
| Stents |
Critical Pulmonary Stenosis (PS)
We generally reserve the term critical PS to describe very severe PV obstruction causing supra-systemic right ventricular systolic pressure, right-to-left atrial level shunt, and/or ductal-dependent pulmonary blood flow (PBF). Initially, prostaglandin E1 (PGE1) infusion should be started followed by percutaneous balloon pulmonary valvuloplasty. In babies with less severe obstruction, balloon valvuloplasty may be performed later, past the neonatal period.
Rubio-Alvarez and Limon-Lason23,24 used a modified ureteral catheter to effect the relief of PV stenosis in early 1950s. In 1979, Semb and colleagues25 reported successful relief of obstruction by forceful withdrawal of an inflated Berman angiographic (BA) catheter across a stenotic PV. In 1982, Kan and associates2 adopted the techniques previously developed by Dotter,26 Gruntzig1 and their colleagues by performing balloon pulmonary valvuloplasty with a double-lumen catheter carrying a nonelastic balloon.2 Static balloon dilatation as described by Kan has become a standard treatment for relief of PV stenosis. Tynan et al19 were the first to report balloon pulmonary valvuloplasty for treatment of critical PS in the neonate. Subsequently other cardiologists27–32 have successfully utilized balloon valvuloplasty to treat the neonates with critical PS.
Balloon Pulmonary Valvuloplasty Procedure
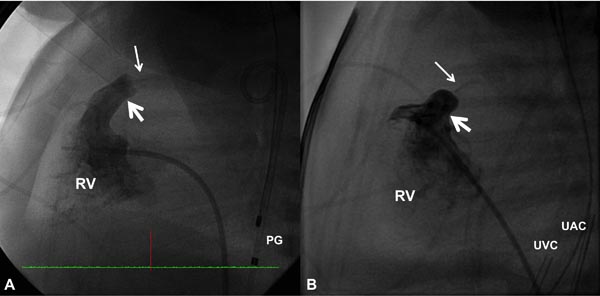
PGE1 is infused to increase the PBF and to improve systemic arterial O2 saturation. Then cardiac catheterization is performed percutaneously via the femoral vein and appropriate hemodynamic data (O2 saturations and pressures) are recorded. Right ventricular (RV) cineangiography in biplane views (sitting-up and lateral) is carried out and PV annulus is measured in both views and averaged. Measurements obtained both by echocardiogram and angiocardiogram are used to decide on the diameter of the PV annulus. RV cine in a straight lateral view is most useful in this regard (Figure 20.1).
Figure 20.1. Selected RV cineangiographic frames in lateral view of 2 neonates with critical PS with intact ventricular septum showing extremely narrow contrast jets (thin arrows). The valve annulus is marked with thick arrows. PG, pigtail catheter; UAC, umbilical artery catheter; UVC, umbilical venous catheter.
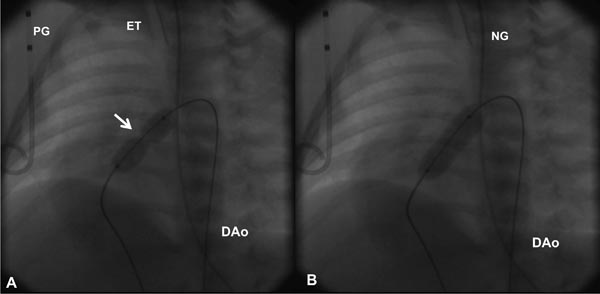
Based on the cardiologist’s preference, a right coronary artery (Cordis), angled Glidecath (Meditech), or cobra (Cook) catheter is positioned in the right ventricular outflow tract (RVOT) and a floppy-tipped coronary guide wire (GW) is advanced across the PV and from there into either branch of PA or into the descending aorta (DAo) via the patent ductus arteriosus (PDA) (Figure 20.2); the latter is ideal because of the increased stability of GW which facilitates positioning an appropriately sized balloon catheter across the PV. The catheter in the RVOT is advanced across the PV into the DAo (or branch PA, as the case may be). The GW is then replaced with a GW that is suitable to place the selected balloon angioplasty catheter. Although, the initial suggestions33–35 were to employ balloons 1.2 to 1.4 times the PV annulus, current recommendations36–38 are that we limit the size of a balloon from 1.2 to 1.25 times the valve annulus. The selected balloon dilatation catheter is advanced over the GW already in place and positioned across the PV. A frozen video frame of the RV cineangiogram is put on view on the screen, and bony landmarks such as ribs, sternum, or other fixed landmarks are used to position the center of balloon across the PV annulus. The balloon is inflated with mixture of contrast material (X1) and saline (X4) by means of any commercially available inflators; the pressure of inflation is adjusted up to the manufacturer-recommended pressure (less than burst pressure) or until the disappearance of the balloon waist (see Figure 20.2). If the balloon is not correctly centered across the PV, the location of the catheter is adjusted and the balloon is inflated again. After satisfactory balloon inflation is attained, one more balloon inflation is performed. Use of adenosine39 or right ventricular pacing40 is not necessary in neonates to achieve good position of the balloon. The balloon catheter is withdrawn and a multipurpose catheter is advanced over the GW already in place. Pressure pullback across the PV is recorded to document the results of balloon valvuloplasty. We also obtain blood samples from the PA, superior vena cava (SVC), and aorta (Ao) to calculate the cardiac index. After recording postballoon hemodynamic data, right ventricular angiography is performed to demonstrate improved flow across the PV (Figures 20.3 and 20.4).
Figure 20.2. Selected cinefluorographic frames in a sitting-up (15° left anterior oblique with 35° cranial angulation) view demonstrating a balloon angioplasty across the stenotic PV with waisting of the balloon (arrow) during the initial phase of balloon inflation (A). The waist has completely disappeared with further balloon inflation (B). Note the GW is passing through the ductus into the DAo. ET, endotracheal tube; NG, nasogastric tube; PG, pigtail catheter.
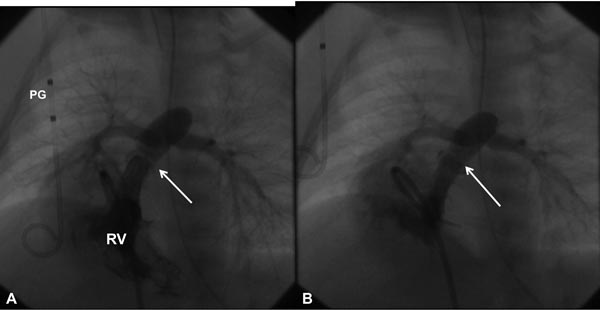
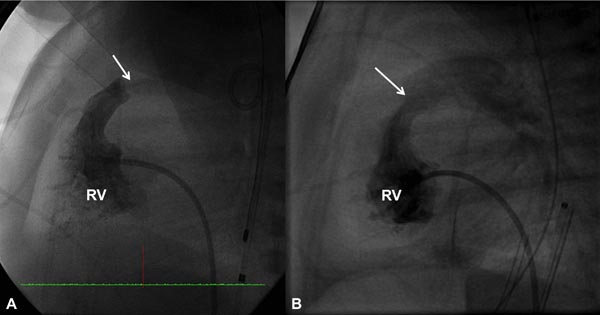
Figure 20.3. Selected RV cineangiographic frames in a sitting-up view before (A) and immediately following (B) balloon pulmonary valvuloplasty. Note the thin jet of contrast (arrow) across the thickened and domed PV prior to valvuloplasty (A). The jet width has markedly increased following valvuloplasty (B). PG, pigtail catheter.
Figure 20.4. Selected RV cineangiographic frames in a lateral view prior to (A) and immediately following (B) balloon pulmonary valvuloplasty. Note the thin jet of contrast (arrow) across the PV prior to valvuloplasty (A) has markedly increased (arrow) following valvuloplasty (B). PG, pigtail catheter.
If it is not possible to advance an appropriately sized balloon catheter across the severely stenotic PV, initial predilation is performed with smaller, 3- to 4-mm diameter balloon catheters (balloon catheters used for coronary angioplasty). This is followed by appropriately sized balloon catheter (Figure 20.5).
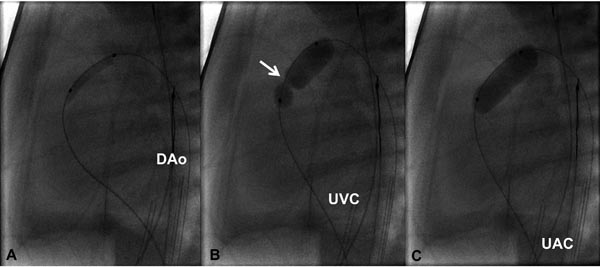
Figure 20.5. Selected cinefluorographic frames in a lateral view illustrating the use of progressively larger balloons in a one-day-old baby with critical PS. A coronary GW was positioned across the PV and across the ductus into the DAo. A 3.5-Fr catheter carrying 3-mm diameter balloon was used to dilate the PV (A); this is followed by a 6-mm (B) diameter balloon. Waisting of the balloon in the initial phases of balloon dilatation (arrow in B) was completely abolished (C) on further inflation of the balloon. UAC, umbilical artery catheter; UVC, umbilical venous catheter.
On exceptional circumstances, especially when associated with hypoplastic RV or in the existence of severe infundibular stenosis (IS), it may not be possible to cross the PV using an anterograde approach. In these situations, the GW and the balloon catheter may be positioned across the PV in a retrograde fashion from the Ao via the ductus and the PA into the RV across the PV. Figure 20.6 demonstrates one such example from our case material.
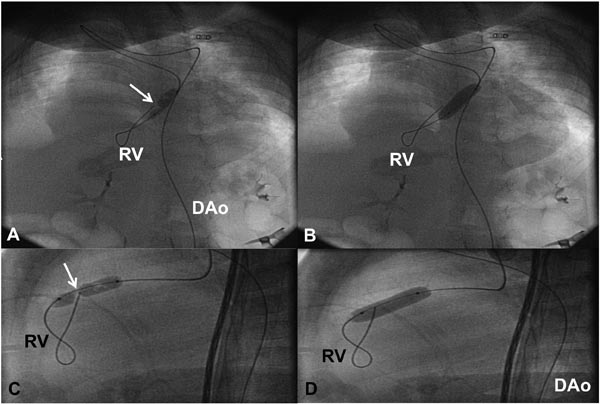
Figure 20.6. In a neonate with critical PS in whom we were unable to pass any catheter or GW across the PV using an anterograde approach, the GW and balloon dilatation catheter were introduced via retrograde approach from the Ao through the ductus and the PA into the RV across the PV. Selected cinefluorographic frames in sitting-up (A and B) and lateral (C and D) views demonstrating waisting of the balloon during the initial phase of balloon inflation (A and C) and complete disappearance of the waist with further balloon inflation (B and D). DAo, descending aorta.
Results
Immediate improvement is demonstrated by reduction in pressure gradient across the PV, decrease in right ventricular peak systolic pressure and RV to aortic systolic pressure ratio, and increased flow across the RVOT (see Figures 20.3 and 20.4) by angiography.21,27–32 Following successful balloon dilatation, extubation and stopping of PGE1 infusion can be accomplished in most babies. Nevertheless, some babies do not tolerate discontinuing PGE1 infusion because they may develop severe arterial desaturation; this is thought to be related to right-to-left shunt across the patent foramen ovale (PFO). Nearly 25% patients29,30 may need PGE1 for 3 to 21 days following balloon pulmonary valvuloplasty. Right-to-left shunt across the PFO mentioned above may be related to persistence of valvar stenosis, development of IS, or decreased compliance of the RV. Persistence of valvar stenosis may be dealt with repeat balloon valvuloplasty or surgical valvotomy. IS may be addressed with beta-blocker therapy. In our experience, the persistence of hypoxemia is secondary to decreased right ventricular compliance. A few of these infants may need prolonged administration of intravenous PGE1. Alternatively, aortopulmonary shunt may be performed to maintain adequate pulmonary flow. Deployment of stents into the ductus is another option.41,42 However, experience with the use of ductal stents is limited, but it can be performed.41,42
A number of studies showed good intermediate-term results, and these are similar to surgical outcomes.29,30,43 For instance, remodeling of the RV and proper growth of all 3 components of the RV were demonstrated by Tabatabaei and associates.30 The residual trans-PV pressure gradients were low at 15 ± 9 mmHg at 6 months to 8 years following balloon pulmonary valvuloplasty. Although the outcome of balloon valvuloplasty is convincingly good, the requirement for re-intervention is greater at 25% compared with that seen in older children at 8% to 10%. This higher re-intervention rate is related to addressing (1) complications associated with the valvuloplasty procedure, (2) hypoxemia due to right-to-left shunt across the PFO, which secondary to decreased right ventricular compliance, and (3) residual obstruction or associated defects.
Critical Aortic Stenosis
The term critical aortic stenosis may be used to describe very severe aortic valve stenosis with a large peak systolic pressure gradient across the aortic valve, signs and symptoms of congestive heart failure (CHF), and/or ductal-dependent systemic circulation. The pressure gradient across the aortic valve may not be high in the presence of poor left ventricular function. Balloon dilatation of the aortic valve is considered an acceptable option for surgical aortic valvotomy in the management of critical aortic obstruction in the newbon.44,45 Following initial supportive therapy, including administration of PGE1 as necessary, transcatheter balloon aortic valvuloplasty is performed. In babies with less severe obstruction, balloon aortic valvuloplasty may be undertaken later, beyond neonatal period.
Following successful adaptation of Gruntzig’s technique1 to PV stenosis2 and aortic coarctation3,4,46 by the respective investigators, Lababidi and his colleagues5,6 employed balloon dilatation technique to alleviate aortic valve obstruction. He subsequently extended the technique to the neonate with critical aortic valve stenosis.20 At first, balloon aortic valvuloplasty was performed percutaneously via retrograde femoral arterial route.20,22,44,45 Since there is a potential for development of femoral artery occlusion, other methods of catheter entry such as carotid,47 axillary,48 umbilical,49 or subscapular50 artery and anterograde femoral venous51,52 as well as anterograde, transumbilical venous53,54 routes of catheter entry were tried in an attempt to accomplish balloon aortic valvuloplasty. Our own choice is to use anterograde, transumbilical venous route53,54 and, if not successful, retrograde femoral arterial route is used. Retrograde transumbilical arterial, anterograde femoral venous, and carotid artery cut-down are the other available choices.
Balloon Aortic Valvuloplasty Procedure
Each of the above-mentioned methods will be reviewed briefly.
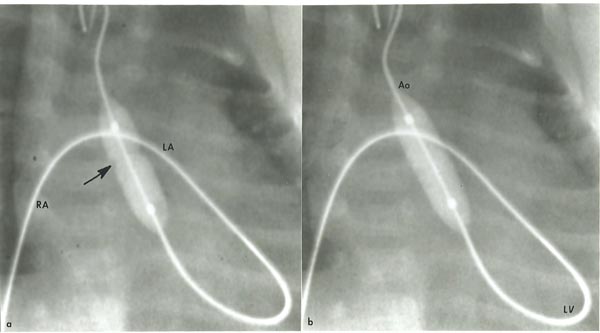
Transumbilical venous balloon aortic valvuloplasty As mentioned in Chapter 19, we encourage our neonatologists to place an umbilical venous catheter (UVC) very early and position the tip of the catheter in the right atrium (RA), prior to closure of the ductus venosus (DV). At the time of balloon aortic valvuloplasty, the UVC is replaced over a GW with a 5-Fr sheath and the sheath tip positioned in the low RA. Following recording the usual catheterization data and left ventricular angiography, the aortic annulus diameter is measured in multiple views. This along with echocardiographic diameter is used to estimate the aortic annulus diameter. A 4-Fr multi-A2 catheter (Cordis) with a slightly curved tip (special order) or a similar catheter is advanced via the umbilical venous sheath into the left atrium (LA) across the PFO and then across the MV into the left ventricle (LV). With the assistance of a J-shaped and/or a straight, soft-tipped 0.035 inch Benston GWs (Cook), the catheter is advanced into the ascending aorta (AAo) and if feasible, the catheter tip is positioned in the proximal part of the DAo. At this point of time, the GW is replaced with a 0.018 or 0.025 inch J-tipped Amplatz extra stiff wire (Cook). A 6- to 8-mm diameter Tyshak II (Braun) or ultrathin (Meditech) balloon angioplasty catheter is advanced over the GW already in place from the RA, LA, LV, and Ao, while preserving a wide loop of the GW in the LV. The diameter of the selected balloon should be 0.8 to 1.0 times the aortic valve annulus. After the balloon is positioned across the aortic valve, the balloon is inflated with diluted contrast material up to the manufacturer’s suggested pressure, or until the balloon waist is abolished (Figure 20.7). The balloon inflation is repeated once or twice to ensure adequate valvuloplasty. Then the balloon angioplasty catheter is exchanged with a 4-Fr multi-A2 (Cordis) catheter leaving its tip positioned in the Ao and the GW removed. Following aortic root angiography, a pressure pullback across the aortic valve is recorded. Angiogram from the LV is optional. Heparin is given during the procedure and activated clotting times (ACTs) monitored. Vancomycin is administered for antibiotic prophylaxis because of extensive handling of the umbilical area during valvuloplasty procedure.
Figure 20.7. Selected cinefluorographic frames demonstrating the position of the balloon across the aortic valve introduced anterogradely from the umbilical vein, RA, LA, LV and Ao. Note the waist (arrow) of the balloon (a) which was completely abolished after further inflation of the balloon (b). Reproduced with permission from Neonatology Today 2007;2(10):1–12.
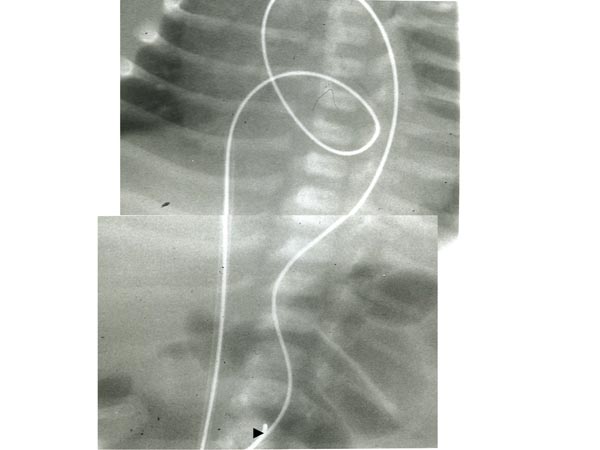
At times, the operator may not be able to maneuver the GW into the DAo or position the balloon catheter across the aortic valve despite multiple attempts. In such circumstances, a gooseneck micro-snare (Microvena, White Bear Lake, MN) may be positioned in the DAo via the umbilical or femoral artery and advanced into the aortic arch and the tip of the anterogradely placed GW is snared and pulled down into the DAo and held in place (Figure 20.8). Thus, an umbilical venous-to-umbilical/femoral arterial wire “rail” is created. With a gentle traction on the umbilical/femoral artery component of the rail (while preserving the wire loop in the LV), the balloon angioplasty catheter may be positioned easily anterogradely across the aortic valve and balloon aortic valvuloplasty undertaken. After completing the procedure, the GW is released from the snare and removed via the umbilical vein; the existence of a catheter over the whole course of the GW within the heart guards the intracardiac structures from injury.53
Figure 20.8. Selected cinefluorographic frames showing GW rail from the umbilical vein, RA, LA, LV, ascending Ao and DAo. Note the snare (arrowhead at the bottom) holding the wire. Reproduced with permission from Neonatology Today 2007;2(10):1–12.
Retrograde femoral arterial balloon aortic valvuloplasty The technique of balloon aortic valvuloplasty is similar to that used in older infants and children, described in detail elsewhere.55,56 In brief, a 4-Fr sheath is inserted into the femoral artery percutaneously and a 4-Fr multipurpose catheter is advanced into the AAo. A 0.014 inch, floppy-tipped coronary GW or a 0.035 inch straight Benston GW (Cook) is advanced into the LV across the aortic valve and the catheter advanced over the wire into the LV. After recording the left ventricular pressure, I usually exchange this catheter with a 4-Fr pigtail (PG) catheter and perform an LV cineangiogram (Figure 20.9
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree