CATHETER INTERVENTIONS IN THE NEONATE:
PART I. NONSURGICAL ATRIAL SEPTOSTOMY
Introduction
While the seeds of interventional pediatric cardiology were planted in the 1950s by Rubio-Alvarez and Limon-Lason by their efforts on percutaneous pulmonary and tricuspid valvotomy,1,2 by Dotter and Judkins in the early 1960s by their gradational vascular dilatation techniques,3 by Rashkind and Miller in the mid-1960s by balloon atrial septostomy,4 by Porstmann et al in the late 1960s by transcatheter closure of patent ductus arteriosus (PDA),5,6 and by King and Mills in the mid-1970s by nonsurgical closure of atrial septal defects (ASDs),7,8 it was not until Kan and her associates9 extended Gruntzig’s technique10 of balloon angioplasty for pediatric use in the early 1980s did transcatheter therapy in children become a reality.11–15 These techniques were adapted gradually to treat neonates with congenital heart defects (CHDs).16–20
In this and the subsequent 2 chapters, a variety of catheter interventional techniques (Table 19.1) that are presently being used in young babies will be reviewed; in this chapter, nonsurgical atrial septostomy will be discussed. The other techniques enumerated in Table 19.1 will be addressed in Chapters 20 and 21.
Table 19.1. Catheter interventional techniques used in the neonate
| Nonsurgical atrial septostomy |
| Balloon angioplasty/valvuloplasty |
| RF perforation of the atretic PV |
| Transcatheter occlusion of shunts |
| Stents |
Rashkind and Miller4 described a balloon atrial septostomy technique in 1966; this is now called Rashkind’s balloon atrial septostomy or Rashkind’s septostomy for short. This procedure was extensively used to increase mixing at the atrial level in neonates with transposition of the great arteries (TGA). This technique was subsequently extended to many other CHDs in which enlarging the atrial defect is valuable, reviewed elsewhere.21 Park and his associates extended the utility of balloon septostomy in the mid-to-late 1970s, by initiating blade atrial septostomy to enlarge defects surrounded by thick atrial septae.22 A retractable blade (knife) is built within the catheter, which when positioned appropriately can cut (incise) the lower margin of the patent foramen ovale (PFO); this is followed by Rashkind’s balloon atrial septostomy. Subsequently, balloon angioplasty (static balloon dilatation),21,23,24 stents,25,26 Ross trans-septal puncture,27 radiofrequency (RF) ablation,28–30 and cutting balloons30 were introduced to create and/or enlarge defects in the atrial septum.
Septostomy procedures (Table 19.2) will be described first, followed by a review of cardiac defects in which atrial septostomy is likely to be beneficial.
Table 19.2. Septostomy procedures
| Rashkind balloon atrial septostomy |
| Blade atrial septostomy |
| Balloon angioplasty |
| Atrial septal perforation |
| Stent implantation |
Catheter Interventional Procedures for Atrial
Septostomy Rashkind Balloon Atrial Septostomy
Since the diagnosis of TGA can be made with ease by echocardiographic studies at the present time (see Chapters 11 and 28), the usual hemodynamic data and cineangiography are not necessary for confirming the diagnosis. However, aortic saturation and pressure pullback across the atrial septum and echocardiographic size of atrial defect are documented. In babies with other diagnoses, appropriate data to define the problem should be secured. A balloon septostomy catheter (BSC) is inserted via a sheath placed percutaneously in the femoral vein and advanced into the left atrium (LA) through the PFO. After ensuring that the catheter tip is positioned in the LA by lateral view fluoroscopy, by advancing the catheter into the pulmonary vein or by echocardiography, the balloon is filled with diluted contrast material to a submaximal amount (usually 2–3 ml) and quickly withdrawn across the atrial septum (Figure 19.1). After the catheter is pulled back into the inferior vena cava (IVC), the catheter is swiftly advanced into the right atrium (RA). All of the above are performed as a single, continuous motion. The balloon is deflated while the catheter is being repositioned into the RA. This jerking motion of the inflated balloon catheter creates a tear in the septum primum (lower margin of the PFO), which is very thin and frail in the neonate. The amount of contrast used to fill the balloon may be increased to 4 ml at the discretion of the cardiologist performing the procedure. We usually perform one more septostomy pull after what is considered adequate septostomy.
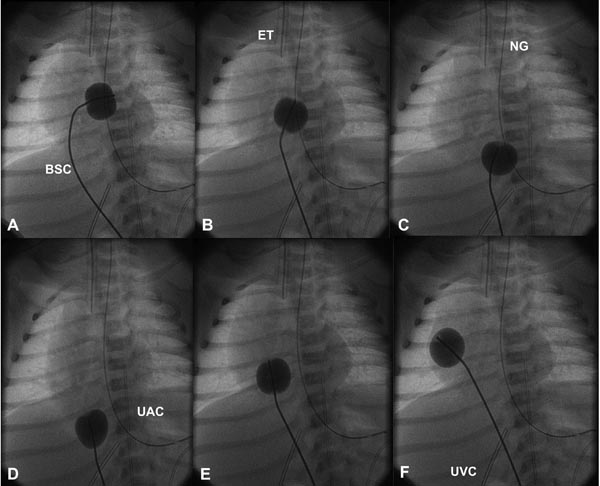
Figure 19.1. Selected cinefluoroscopic frames of the Rashkind balloon septostomy procedure. The balloon is inflated in the LA (A). The BSC is rapidly and forcefully pulled into the RA (B) and IVC (C & D) and rapidly advanced back into the RA (E & F); all of these actions are done as a single motion. Rapid advancement of the BSC into the RA (E & F) is done to avoid inadvertently occluding the IVC in case of failure to deflate the balloon (which is quite rare). At about the same time the balloon is deflated. ET, endotracheal tube; NG, nasogastric tube; UAC, umbilical arterial catheter; UVC, umbilical venous catheter.
Improvement in systemic arterial oxygen saturation, reduction, or absence of pressure gradient across the PFO and increase in the echographic size of the defect in the atrial septum (Figure 19.2) with laminar Doppler flow across the atrial septum (Figure 19.3) are usually seen following successful septostomy. In babies in whom atrial septostomy is undertaken to relieve interatrial obstruction, pressure gradient across the atrial septum is decreased or abolished. Balloon sizing of the atrial defect both before and after balloon septostomy is another method of evaluation of result of the septostomy and is routinely performed by some cardiologists.
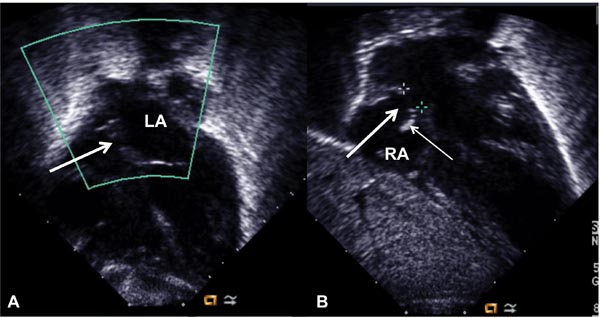
Figure 19.2. Selected video frames from subcostal views showing a very small interatrial opening (arrow in A), which became larger (arrow in B) following balloon atrial septostomy. Thin arrow in B shows the torn flap of the lower margin of the PFO. LA, left atrium; RA, right atrium.
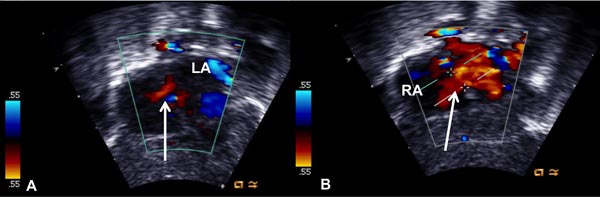
Figure 19.3. Selected video frames from subcostal views showing a very small color flow jet across the interatrial opening (arrow in A), which became larger (arrow in B) following balloon atrial septostomy. LA, left atrium; RA, right atrium.
When Rashkind and Miller4 first described balloon atrial septostomy, they introduced the catheter into the femoral vein by the cutdown approach. To avoid femoral venous cutdown, some cardiologists advocated insertion of the catheter via the umbilical vein.31 When percutaneous catheterization by Seldinger technique32 was adapted to pediatric practice, it was possible to insert the balloon catheter via femoral venous sheaths.33,34
Our personal preference is to carry out balloon atrial septostomy via the umbilical venous route. Consequently, we persuade our neonatology colleagues to place an umbilical venous catheter very early and advance the tip of the catheter into the RA, prior to constriction of the ductus venosus. At the time of septostomy, the umbilical venous catheter is replaced over a guide wire with a sheath of suitable size.
The practicability of undertaking balloon septostomy bedside with echo guidance has been established.35,36 However, most cardiologists, including the author of this chapter, prefer to perform the procedure in the catheterization laboratory.
The catheters initially used for septostomy were Rashkind BSCs manufactured by USCI, Boston, MA. Since the catheters were straight, it has been difficult to advance these catheters into the LA in some cases. Moreover, these balloons would take only a limited volume of contrast that may not be sufficient for adequate septostomy. Consequently, most cardiologists have changed to Edwards septostomy catheters, manufactured by American Edwards/Baxter Healthcare Corp., McGaw Park, IL. There is a gentle curve at the tip of the catheters, which facilitates easy entry into the LA. As well, a larger volume of contrast material may be used to inflate these balloons. Subsequently, atrioseptostomy catheters manufactured by B/Braun, Bethlehem, PA have become available for clinical use. It should be mentioned that there are no reported data comparing the effectiveness of all the available balloon catheters. For this reason, the choice of the type of catheter utilized for septostomy is largely at the discretion of the cardiologist performing the procedure.
Blade Atrial Septostomy
In some conditions such as hypoplastic left heart syndrome (HLHS) (as well as in older patients), the lower margin of the PFO is thick and may not be ruptured by Rashkind balloon septostomy. The procedure may simply stretch and not tear the lower margin of the PFO. To circumvent this problem, Park and his associates22 designed catheters with built-in blade (knife) (Cook Inc., Bloomington, IN). Three catheters with different blade sizes are available through the manufacturer. The smallest size catheter available is selected for the neonate and is positioned across the PFO. The location of the tip of the catheter (in the LA) is established and the blade is opened. The blade is pointed anteriorly and to the left (Figure 19.4) and the catheter is gradually withdrawn (in contrast to the swift, jerky motion of balloon septostomy). This should incise the lower margin of the PFO. The procedure is repeated one or two more times with slightly varying angulations of the blade. Balloon septostomy should follow. Assessment of the results is similar to that described in the preceding section. A 70% and 90% success rate37–39 is anticipated.
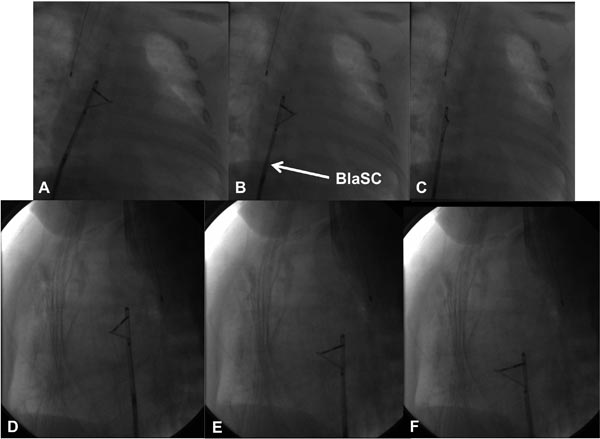
Figure 19.4. Selected cinefluoroscopic frames of the blade septostomy procedure posteroanterior (A, B, and C) and lateral (D, E, and F) views. Note the position of the blade is pointing to left and anteriorly. In contrast to the very rapid pullback in the Rashkind balloon septostomy procedure, blade septostomy procedure is performed by slow withdrawal of the blade septostomy catheter (BlaSC).
Balloon Angioplasty
Experimental static balloon dilatation of the atrial septum in animal models was reported in an abstract form by Mitchell40 and Sideris41 and their colleagues, but to the best of my knowledge, human application of this technique has not been reported by these investigators. Shrivastava and her associates23
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree