Catheter Interventions in Congenital Heart Disease
Robert H. Beekman III
A wide array of percutaneous transcatheter therapies is available to treat congenital cardiac malformations. Patients of all ages, from the fetus through adults, benefit from these minimally invasive therapies. This chapter describes the transcatheter procedures available to the interventional cardiologist for treatment of many common congenital heart defects. Balloon dilation of stenotic valves and vessels and the use of implantable devices, such as embolization coils, septal occluders, and stents, are reviewed. Newer technologies that promise major advances in transcatheter therapy for congenital heart disease are also briefly discussed.
Overview and Historical Perspective
Pediatric interventional cardiac catheterization was pioneered by William Rashkind (1) in 1966, when he first described the technique of balloon atrial septostomy in infants with transposition; the procedure now carries his name. The next major advance occurred in 1982, when Kan (2) described percutaneous balloon dilation in children with pulmonary valve stenosis. During the 1980s, catheter-based therapies were developed for a variety of congenital cardiac defects including pulmonary valve stenosis, pulmonary artery stenosis, aortic valve stenosis, coarctation of the aorta, patent ductus arteriosus (PDA), and secundum atrial septal defect (ASD). By 1990, interventional cardiology was established as a mature subspecialty of pediatric cardiology.
Transcatheter therapies are now available for 30% to 40% of patients with congenital heart disease. Percutaneous balloon dilation is the treatment of choice for congenital pulmonary valve stenosis, aortic valve stenosis, pulmonary artery stenosis, and postoperative recurrent coarctation. Balloon-expandable stent implantation has proven invaluable for treating pulmonary artery stenosis and coarctation. Occlusion devices implanted percutaneously are standard therapy for PDA and for the secundum ASD. Promising clinical trials are under way to evaluate the effectiveness of transcatheter-delivered occlusion devices as alternatives to surgery in children with ventricular septal defects (VSDs), of the muscular or perimembranous types. Even more novel interventions are being evaluated, the most promising being percutaneous transcatheter implantation of valves in the right ventricular (RV) outflow tract.
This chapter provides a brief overview of the field of interventional pediatric cardiology. The most common transcatheter therapies are discussed, with a focus on their indications, contraindications, short- and long-term outcomes, and associated complications. Because interventional pediatric cardiology encompasses a large number of procedures for a wide variety of cardiac defects, the discussion of each procedure is, by necessity, brief.
Balloon Dilation for Congenital Aortic Valve Stenosis
Balloon dilation of a congenitally stenotic aortic valve was first described by Lababidi in 1983 (3). The procedure has since been evaluated in numerous pediatric centers (4,5,6,7,8,9). Percutaneous balloon dilation provides good short- and intermediate-term relief of obstruction in patients with congenital aortic valve stenosis. There have, however, been no prospective trials comparing balloon dilation with surgical aortic valvotomy. Nevertheless, there is widespread agreement that the effectiveness of balloon dilation compares favorably to that of surgery, specifically regarding gradient relief and the occurrence of important valve regurgitation. Thus, percutaneous balloon dilation has been adopted by most pediatric cardiology programs as the treatment of choice in this group of patients.
Indications and Contraindications
Indications for balloon aortic valvotomy parallel those previously adhered to for surgical valvotomy. Some centers have relaxed their clinical indications because of the perception that the risk-to-benefit ratio of a percutaneous procedure is probably superior to that of surgery. Currently, in our program, the indications for balloon valvotomy in patients with congenital valvar aortic stenosis are (a) peak systolic gradient greater than 65 mm Hg in an asymptomatic child; (b) peak systolic gradient of 45 to 65 mm Hg in a child with electrocardiographic evidence of myocardial ischemia (at rest or during exercise) or with symptoms attributable to myocardial ischemia; and (c) the presence of critical aortic stenosis in an infant with low cardiac output, regardless of gradient.
Contraindications to balloon valvotomy include (a) mild to moderate aortic valve stenosis (peak systolic gradient <45 mm Hg) in an asymptomatic child, (b) the presence of associated severe aortic regurgitation (of sufficient severity to warrant valve repair or replacement), (c) important hypoplasia of the aortic valve annulus (e.g., <5 mm in a newborn), and (d) aortic valve calcification (rare in childhood).
Technique
The procedure is most often performed using a retrograde transarterial approach. In newborns, we often use a transumbilical arterial approach (10), to spare the newborn femoral artery. Once arterial access is obtained, the stenotic valve is crossed in retrograde fashion. A soft-tipped wire must be used in newborns to avoid perforating an aortic valve cusp. A balloon is utilized with an inflated diameter of 90% to 100% of the diameter of the valve annulus. In children in whom concern exists for femoral artery injury, a double-balloon technique can be used to avoid the larger catheter required with single-balloon dilation (Fig. 83.1). Throughout the procedure, left ventricular (LV) pressure is monitored by an antegrade catheter placed across the foramen ovale or through a transseptal puncture. Patients are fully heparinized. The balloon (or balloons, in the case of a double-balloon technique) is inflated briefly by hand, with care taken to observe the waist of the stenotic valve near the midportion of the balloon. After dilation, repeat hemodynamic measurements are obtained, and an aortic root angiogram is performed to document the presence and severity of any aortic regurgitation that may have been produced.
Short- and Long-Term Results
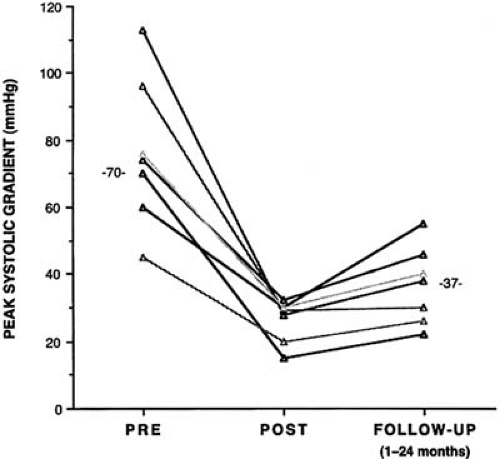
The largest study describing the acute outcome after balloon dilation in children with congenital aortic stenosis was reported by the Valvuloplasty and Angioplasty of Congenital Anomalies (VACA) Registry (9) in 1990. The VACA Registry reported information from 204 children whose short-term data were submitted to the registry after balloon dilation procedures performed between 1982 and 1986. The registry made no attempt to standardize the dilation procedure. The patients ranged in age from newborn to young adult, with a mean age of 10.1 years. All had congenital valvar aortic stenosis of moderate to severe degree. Balloon dilation acutely decreased the peak systolic gradient from 77 to 30 mm Hg (i.e., mild), with a 10% incidence of increased aortic valve regurgitation. In our experience, less satisfactory gradient relief typically indicates either a suboptimal technical procedure or the presence of more complex LV outflow tract disease (i.e., annulus hypoplasia, subvalvular stenosis, or calcific stenosis).
The longer-term results of aortic valve balloon dilation for congenital aortic valve stenosis indicate that, as with surgical valvotomy, the procedure is palliative. Several studies (11,12,13) have shown satisfactory gradient relief for a period of 2 to 5 years, and longer-term benefits can be expected in some patients. Shim et al. (14) noted that successful balloon aortic valvuloplasty was effective in decreasing LV mass in children with isolated aortic stenosis. In this small study, the decrease in LV mass was gradual and was not statistically significant until the second year.
Neonates
Neonates with critical aortic stenosis constitute a high-risk subgroup that deserves special mention (Fig. 83.2). Newborns with critical aortic stenosis present with severe LV dysfunction and shock, and they must be resuscitated with prostaglandin E1 and inotropic support. As with older children, balloon dilation is an effective palliative therapy in newborns with critical aortic stenosis (10,15,16,17) (Fig. 83.3). Egito et al. (16) described the results of balloon dilation in 33 neonates ranging in age from 1 to 33 days. The procedure was performed from the
umbilical artery in 11, from the femoral artery in 20, and antegrade across a patent foramen ovale in 2 children. A second valve procedure was required in 12 children during the follow-up period, underscoring the palliative nature of this procedure. Mosca et al. (18) compared the results of neonatal balloon dilation with those of surgical valvotomy for critical aortic stenosis and found no significant difference between the procedures in the degree of gradient reduction, aortic regurgitation, or patient survival.
umbilical artery in 11, from the femoral artery in 20, and antegrade across a patent foramen ovale in 2 children. A second valve procedure was required in 12 children during the follow-up period, underscoring the palliative nature of this procedure. Mosca et al. (18) compared the results of neonatal balloon dilation with those of surgical valvotomy for critical aortic stenosis and found no significant difference between the procedures in the degree of gradient reduction, aortic regurgitation, or patient survival.
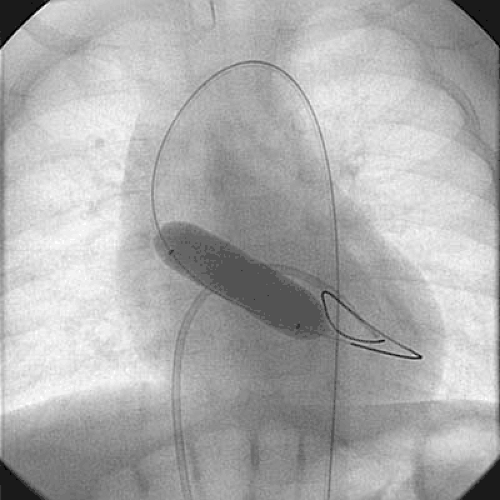 FIGURE 83.2. Aortic valve dilation in a newborn with critical aortic stenosis. An antegrade catheter is positioned in the left ventricle via the patent foramen ovale. |
Complications
Beyond the newborn period, balloon aortic valvotomy is a relatively safe procedure. Immediate complications include aortic valve regurgitation, iliofemoral artery injury, hemorrhage, ventricular arrhythmia, endocarditis, valve leaflet or ventricular perforation, injury to the anterior mitral leaflet, and, rarely, death. Death has been exceedingly uncommon outside the newborn period. In the VACA Registry (9), aortic regurgitation reportedly occurred in only 10% of the children studied. More recent experience suggests that balloon dilation produces new aortic insufficiency in 25% to 30% of patients, and it is typically mild. Severe aortic insufficiency occurs in approximately 5% of patients after the procedure. Aortic insufficiency may relate to a balloon-to-annulus ratio that exceeds 1.0 (9) or to valve morphology (6). Injury to the iliofemoral artery most frequently occurs in infants younger than 12 months of age. For this reason, we prefer the transumbilical route whenever possible for balloon dilation in newborns with critical aortic stenosis (10).
Complications are substantially more frequent and more severe in neonates. The mortality in newborns with critical aortic stenosis is substantial and ranges from 10% to 20% (16,18). Other important complications in the newborn include LV perforation with wire or catheter, severe aortic insufficiency (at times owing to inadvertent valve leaflet perforation and subsequent dilation), arrhythmia, hemorrhage, sepsis, and endocarditis. To avoid sepsis or endocarditis, vancomycin is administered to all patients undergoing transumbilical dilation procedures.
Balloon Dilation of Congenital Pulmonary Valve Stenosis
Percutaneous balloon valvotomy was first performed for pulmonary stenosis. In 1979, Semb (19) reported a dynamic technique in which a balloon was withdrawn across the stenotic pulmonary valve. In 1982, Kan (2) reported the first static balloon dilation in children with pulmonary valve stenosis; this technique is still used today. After these early reports, many institutions confirmed the safety and efficacy of percutaneous balloon dilation for congenital pulmonary valve stenosis. The technique typically reduces the peak systolic valve gradient to approximately 15 to 25 mm Hg, which represents mild, clinically insignificant pulmonary stenosis. With the exception of neonates, pulmonary valve dilation is associated with few complications. The procedure has clearly replaced surgical valvotomy as the treatment of choice in congenital pulmonary valve stenosis (20




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree