52 Catheter-based Treatment of Congenital Heart Disease
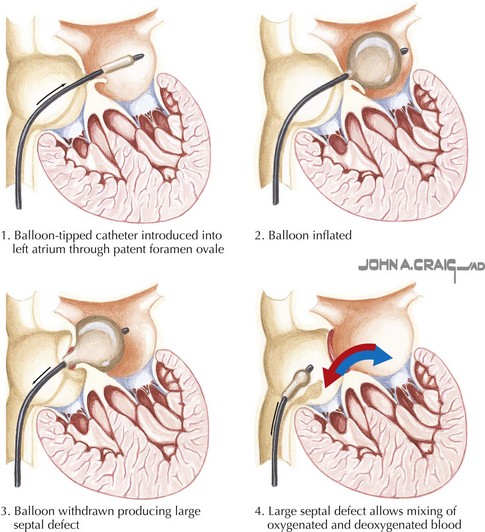
Balloon Atrial Septostomy
The procedure described by Rashkind remains the basis of balloon atrial septostomy as it is performed today, albeit with advances in imaging and catheter design. A large balloon is passed transvenously across the foramen ovale and pulled forcefully across the atrial septum, tearing the thin tissue in the floor of the oval fossa and thereby creating a larger atrial septal defect (ASD) and improving intracardiac mixing and systemic oxygen delivery (Fig. 52-1). Although prostaglandin and early surgery have reduced the need for balloon atrial septostomy in many infants, it remains a lifesaving palliative procedure for some. Indeed, today balloon atrial septostomy is routinely safely and effectively performed at the bedside under echocardiographic guidance.
Balloon Valvuloplasty
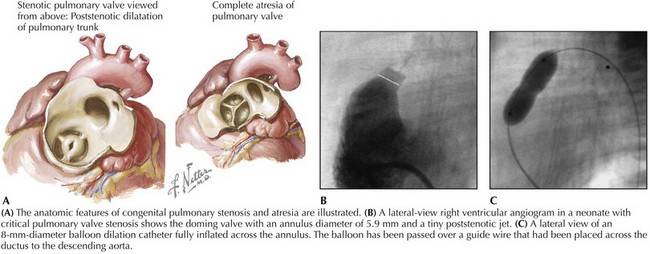
Pulmonary Stenosis
Balloon pulmonary valvuloplasty has become the standard of care for pulmonary stenosis. This procedure is indicated for symptomatic infants and for older children with systolic pressure gradients exceeding 30 mm Hg. After hemodynamics and angiography, an end-hole catheter and wire are placed transvenously across the stenotic valve. A balloon catheter with a diameter 20% to 40% greater than the diameter of the valve annulus is inflated until a “waist” is seen to disappear (Fig. 52-2). Balloon pulmonary valvuloplasty provides similar relief of right ventricular outflow tract obstruction, produces less pulmonary valve insufficiency, has a lower rate of complications when compared to surgery, and is a more comfortable and better tolerated outpatient procedure for the patient. In the neonate with critical pulmonary stenosis, balloon valvuloplasty is more technically challenging and the complication rate is slightly higher, but definitive results are achieved in most patients. The long-term outcome is excellent except occasionally in patients with dysplastic pulmonary valves or in neonates with a hypoplastic valve annulus. The procedure can also be applied in patients with pulmonary atresia with an intact ventricular septum after initial perforation of the atretic valve.
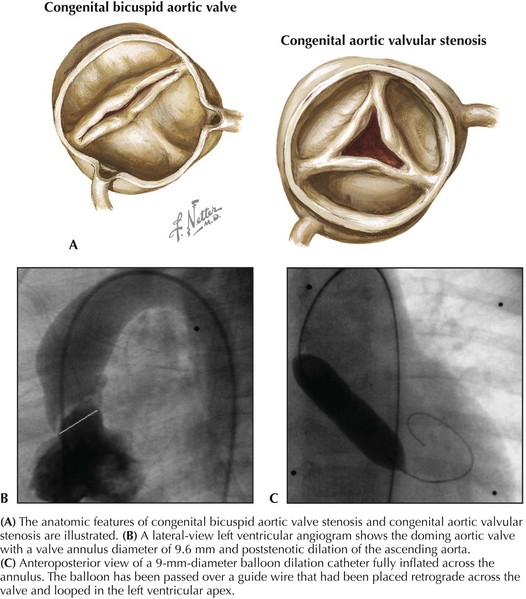
Aortic Stenosis
Balloon aortic valvuloplasty is considered the initial treatment of choice for congenital aortic stenosis, albeit a palliative procedure that most commonly will have to be followed by a subsequent intervention at an older age. Symptomatic infants with critical aortic stenosis and older children with congenital aortic stenosis and peak systolic pressure gradients exceeding 60 mm Hg are candidates for intervention. The valve is usually crossed retrograde, and an exchange wire is looped in the left ventricular apex. A balloon catheter with a diameter 90% to 100% of the diameter of the valve annulus is inflated several times to abolish the waist formed by the valve on the balloon (Fig. 52-3). Reductions of 50% to 70% of the initial pressure gradient are typical. Many patients require repeat intervention within 5 to 10 years of undergoing the procedure. Fewer than half will require surgical aortic valve replacement after 10 years of follow-up. Catheter and surgical intervention are equally effective in relieving the obstruction with a similar incidence of important aortic insufficiency, repeat intervention, valve replacement, and mortality.
Balloon Angioplasty and Stent Placement
Pulmonary Artery Stenosis
Balloon-expandable stents represent a major advance over angioplasty in the treatment of pulmonary artery stenosis. The use of stents has improved the acute success rate and results in a greater increase in arterial diameter than does angioplasty alone. The stent is mounted and hand-crimped onto a high-pressure balloon dilation catheter. The stent-balloon complex is then advanced over a wire through a long large-caliber sheath positioned across the stenosis. After positioning of the stent and test angiograms, the stent is expanded by inflating the balloon (Fig. 52-4). The main limitation to the use of stents is patient size. Although modest further expansion of stents is possible later, placing a stent in a small child is technically challenging and often commits that child to surgery in the future. Improvements in stent design have facilitated the delivery of stents to remote sites, but stents less than 6 to 8 mm in final diameter frequently develop stenosis or local thrombosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree