Catheter-based Mitral Valve Procedures: Balloon Mitral Valvuloplasty and MitraClip
Michael S. Kim
John D. Carroll
Part 1. Balloon Mitral Valvuloplasty
Balloon mitral valvuloplasty (BMV) or valvotomy is recommended for symptomatic and some asymptomatic patients with pulmonary hypertension who have rheumatic mitral stenosis (MS). An alternative name for BMV is percutaneous transcatheter balloon mitral commissurotomy and the indications for BMV are analogous to those used for surgical commissurotomy.
Severe MS: A mitral valve area <1.5 cm2, typically producing a resting transmitral gradient well above the 5 to 6 mm Hg range
MS primarily produced by fused commissures
No more than mild (≤2 plus) mitral regurgitation
Before the widespread use of echocardiography, valve morphology was assessed by physical examination. The constellation of findings denoting a patient likely to benefit from commissurotomy include a loud first heart sound, the presence of a crisp opening snap (OS), a short S2-OS interval, and the absence of signs of important mitral regurgitation including an apical systolic murmur and signs of significant left ventricular volume overload.
Echocardiography confirms the diagnosis of MS, characterizes the anatomic factors contributing to obstruction, and rules out contraindications to BMV therapy. The Wilkins score (Table 21.1) is derived from echocardiographic features of the mitral valve and is often predictive of long-term outcome. The score ranges from 4 to 16 by summing four anatomic characteristics each of which receive a grade of 1 to 4, with higher scores
indicative of more extensive disease. A Wilkins score ≤8 portends a favorable long-term result of BMV. The Wilkins score does not include an assessment of commissural fusion.
indicative of more extensive disease. A Wilkins score ≤8 portends a favorable long-term result of BMV. The Wilkins score does not include an assessment of commissural fusion.
TABLE 21.1 Wilkins Echocardiographic Score | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Patients with elevated Wilkins score may undergo BMV if they are at prohibitive risk for open surgery. These MS patients are often elderly, have advanced valve deformities, undergo BMV to achieve goals of palliation of symptoms, and are often treated in the United States or other countries where rheumatic fever has been eradicated for years. The clinical pearls in this group of patients include: (1) the reduction in mitral gradient is often less that those with optimal mitral anatomy for BMV; (2) the risk of producing severe mitral regurgitation remains low, a blessing since surgical valve replacement is not an option; (3) the durability of benefit is often only 2 to 5 years, clearly less than the 10 years or more following BMV in the optimal commissurotomy candidate.
BMV is contraindicated in the following patients:
Surgically eligible patients with extensive mitral valve deformities including extensive subvalvular disease.
Moderate to severe mitral regurgitation.
MS due to mitral annular calcification.
MS due to bioprosthetic valve deterioration.
Left atrial appendage (LAA) or interatrial thrombus.
Clinical pearls have emerged in defining patients in whom BMV is relatively contraindicated due to suboptimal features of the commissures.
If only one commissure is fused since the expanding balloon is less likely to result in splitting.
If either commissure has markedly increased echocardiographic density including calcification since it may not split and the force of the expanding balloon could tear the anterior leaflet away from the annulus.
There are several important preoperative preparations.
Is transseptal catheterization expected to be feasible and straightforward? IVC filters may preclude transseptal catheterization especially if there is evidence of thrombus
or chronic occlusion of the filter. Patients with scoliosis may have distortion of the intrathoracic position of the interatrial septum in relationship to the IVC making transseptal catheterization difficult. Pacemaker leads are not an obstacle to transseptal catheterization but if recently placed they can be displaced if care is not taken. Finally, some patients have a very thick septum that may make puncture difficult. The use of radiofrequency energy may or may not be effective versus needle puncture.
Are there abnormalities of the left atrium that may affect the safety of the procedure? Thrombus in the left atrium must be excluded by transesophageal echocardiography (TEE) before BMV. While care can be taken in manipulating catheters within the left atrium especially by using 3D TEE imaging, inadvertent excursions of a catheter into the LAA may occur and result in systemic embolization. Therefore, finding an LAA thrombus usually results in delaying BMV until full anticoagulation has dissolved the thrombus.
Scheduling the time and location of BMV is important because of the potential complication of inducing severe mitral regurgitation. BMV should be performed in centers that have immediate access to mechanical left ventricular assistance and the ability to convert the patient to open surgery. BMV has not traditionally been performed in hybrid operating rooms because it is possible to stabilize most of these patients who develop severe mitral regurgitation with a combination of intra-aortic balloon counterpulsation and sodium nitroprusside infusion.
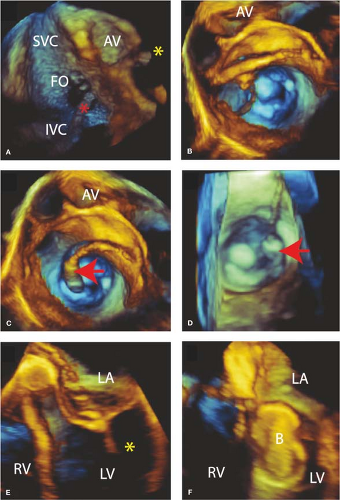
The type of image guidance to be used must be defined as well as the type of anesthesia. There are multiple ways of performing and guiding BMV. Conscious sedation and using only fluoroscopic guidance may be done but the lack of echocardiographic guidance of the transseptal puncture increases the risk of cardiac perforation and tamponade. Centers may utilize intracardiac ultrasound, transthoracic echocardiography, or TEE to visualize the septum, guide transseptal puncture, and use in subsequent steps of the procedure. Echo Doppler has replaced catheter-based assessment of changes in the transmitral gradient and changes in the degree of mitral regurgitation. We have moved to a model of general anesthesia with 3D TEE guidance for most of our patients because of these safety issues as well as the principle that a successful procedure has an anatomical endpoint of as complete of a commissurotomy as possible. This endpoint is best assessed with 3D TEE. Furthermore, using 3D TEE decreases the use of fluoroscopy and this reduces radiation exposure.
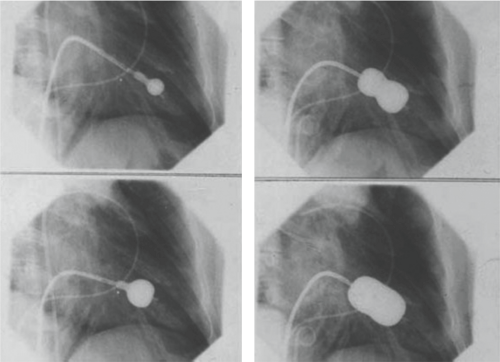
BMV may be performed either in the antegrade or retrograde approach and may utilize either a single- or double-balloon technique. The most common approach, however, is the antegrade approach using the Inoue balloon catheter. Dr. Kanji Inoue, a Japanese cardiac surgeon, developed the Inoue catheter that was first used in 1982. A subsequent clinical trial in the United States and Canada lead to FDA approval. The Inoue balloon is unique in that it is a self-positioning balloon composed of three sections with distinct elastic properties (Fig. 21.1). The balloon catheter allows for both rapid inflation and deflation such that the transient hypotension is not typically sensed in the conscious patient.
The Inoue kit consists of the Inoue balloon catheter, a stretching tube that elongates the balloon at the tip of the catheter, a dilator for dilating the subcutaneous tissue and interatrial septum, a stainless steel guidewire for guiding the insertion of the dilator and Inoue catheter, a stylet that helps direct the Inoue catheter across the valve, and a calibrated syringe used to inflate the balloon to a desired diameter. The 3D TEE guidance to perform these steps is illustrated in Figure 21.2.
The BMV procedure utilizing the Inoue balloon catheter consists of the following steps:
A general diagnostic evaluation (right heart catheterization and possible coronary angiography) may be needed. This is preferably done in the conscious, sedated patient.
A transseptal puncture is used to obtain direct access to the left atrium. This is guided by echocardiography and fluoroscopy. The location of the transseptal puncture on the atrial septum need not be precise but should be located in the fossa ovalis. If a patent foramen ovale is present this may be used with caution since a rigid tunnel can prevent catheter manipulation needed to cross the mitral valve and align it properly.
Following successful transseptal puncture, the patient is anticoagulated (most commonly with heparin) with an ACT of 250 to 300 seconds as the target.
A 12-Fr dilator dilates the interatrial septum.
The guidewire is positioned in the left atrium and used to remove the diagnostic transseptal sheath and to insert the Inoue catheter.
The slenderized Inoue balloon catheter is advanced across the septum into the left atrium over the wire. Care must be taken to not forcefully push the catheter through the septum and cause a tear. A gentle rocking motion is usually effective.
Once the balloon portion of the catheter is across the septum, it is unslenderized by removal of the balloon stretching tube with the subsequent ability to advance the curved and flexible catheter down to the mitral valve.
Crossing the stenotic valve is completed using a combination of fluoroscopic and ultrasound guidance. A stylet is inserted into the central lumen of the Inoue balloon catheter and advanced to the tip. By pulling back the stylet a few inches the catheter moves forward. If the trajectory is correct the catheter passes into the left ventricle.
Before performing balloon inflation, care is taken to be sure the tip of the catheter is freely moving and aligned with the centerline through the valve orifice. Partially inflating the distal portion of the Inoue balloon may assist in determining the central location of the balloon, away from chordal structures.
The balloon is inflated with a calibrated syringe after positioning to insure the central portion of the balloon, the last portion to be fully expanded, is locked on the valve over the fused commissures. During the final part of the inflation sequence there is
often a sudden and distinct expansion of the central portion of the balloon expands corresponding to commissures being split.
After the initial inflation the mitral gradient and the degree of mitral regurgitation are determined using echocardiography and Doppler. If moderate to severe mitral regurgitation has been produced, then the procedure is over. On the other hand if mitral regurgitation is not a problem and a significant gradient persists and the commissures are not completely split then another inflation is performed with a larger volume of the saline-contrast mixture. This is referred to as the step-wise inflation of the balloon starting at an inflation volume that produces a balloon diameter approximately 4 mm less than the nominal diameter.
When the best result has been achieved, that is, maximum reduction in gradient with least increase in mitral regurgitation, the Inoue catheter is removed over the guidewire after slenderizing the catheter.
Before the venous access site is closed it is important to assess the interatrial septum. It is expected that a small atrial septal defect has been created and this typically seals within a few months. Occasionally, a larger defect is created or there is right to left shunting through the defect that causes systemic oxygen desaturation. The defect is then closed with the commercially available septal closure devices.
The venous access site can be closed by manual compression, with or without reversal of the heparin. Alternatively, suture closure techniques can be used.
Videos of the procedure are available online.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree