An electrical storm (ES) is defined as multiple ventricular arrhythmia episodes leading to implantable cardioverter defibrillator interventions. Although conventional rhythm stabilization might be of help acutely, ES involves high mortality and morbidity. We evaluated the effect of catheter ablation strategies in the setting of an interhospital collaborative network on the recurrence of ventricular arrhythmia episodes and mortality in patients with ES. Consecutive patients presenting for invasive treatment of ES from December 2007 to December 2009 were included. All patients underwent catheter ablation of ventricular arrhythmia. The strategies were adapted to the individual cardiac pathologic features. The follow-up examination constituted periodic implantable cardioverter defibrillator interrogation. A total of 32 patients were included. Of the 32 patients, 29 (91%) had monomorphic ventricular tachycardia and 3 ventricular fibrillation. The mean number of implantable cardioverter defibrillator-treated episodes within 7 days before ablation was 16 ± 11. Of the 32 patients, 27 underwent ablation within 24 hours after admission, and 5 underwent acute ablation within 8 hours. In 3 patients, epicardial ablation was performed. In all but 2 patients (6%), the clinical arrhythmia was successfully ablated. During a median follow-up of 15 months, 10 patients (31%) had recurrences of sustained ventricular arrhythmia, including 2 patients (6%) with recurrent ES. Three patients (9%) died during the follow-up period. In conclusion, catheter ablation effectively suppressed ventricular arrhythmia midterm recurrences in patients presenting with ES. Catheter ablation is complex in these severely sick patients. The recurrence rate of ventricular arrhythmia appears to be 31% and the mortality rate to be 9%. Collaborative hospital networks to increase the prompt availability of ES ablation might help to optimize the ES outcome.
Electrical storm (ES) is defined as the occurrence of ≥3 distinct episodes of sustained ventricular arrhythmia triggering implantable cardioverter defibrillator (ICD) treatment within 24 hours. Recent studies have indicated the deleterious effect of the occurrence of ES on the midterm prognosis of these patients, and cardiac death rates of ≤54% within 2 years have been described. The clinical treatment of these patients is challenging and can involve antiadrenergic and antiarrhythmic medication, adequate sedation, or general anesthesia. Furthermore, interventions such as catheter ablation, hemodynamic support systems, or even heart transplantation can be needed. Catheter ablation might help to acutely reduce the arrhythmia burden by eliminating triggers such as ventricular premature beats or modifying the underlying substrate such as myocardial scar regions. Recent studies have focused on the beneficial effect of ES ablation on the midterm prognosis, indicating a reduction in mortality and recurrences of ventricular arrhythmias (including ES).
Methods
The collaborative regional hospital network consists of 7 different hospitals (catchment area of approximately 400,000) that regularly transfer patients to the interventional electrophysiology center. In this network, patients with ES admitted to the collaborating hospitals were included if catheter ablation had been scheduled after contacting the interventional electrophysiology center from December 2007 to December 2009. ES was defined as the occurrence of ≥3 episodes of ventricular arrhythmia separated by >5 minutes during a 24-hour period not due to ischemia. The patients were transferred to the interventional center as soon as they had been stabilized for transportation. After admission, all patients were continuously monitored using 12-lead electrocardiographic monitoring in the intensive care unit to document any clinical arrhythmia. Myocardial ischemia was ruled out as the mechanism for electrical instability. For rhythm stabilization, if recurrent ventricular arrhythmia occurred, conventional strategies using a specified protocol were used as applicable: (1) intensified β-blocker therapy including an intravenous bolus (n = 32); (2) adequate sedation using midazolam (n = 18); (3) additional or intensified antiarrhythmic medication using class I antiarrhythmics and/or amiodarone (n = 24); (4) general anesthesia (n = 5); (5) increased V-pace (n = 4); and/or (6) hemodynamic left ventricular support (n = 3). Cardiogenic shock was defined as prolonged hypotension (>3 hours, <70 mm Hg systolic) under intravenous pressor agents or hemodynamic device support such as an intra-aortic balloon pump or extracorporeal membrane oxygenation.
All patients or family members provided informed consent. The ablation procedure was usually scheduled for the next working day after admission (<24 hours) or on the day or night after admission if no temporary rhythm stabilization was achieved (acute ablation). The patients were studied using an electroanatomic 3-dimensional mapping system (CARTO XP, CARTO-3, Biosense Webster, Diamond Bar, California, and NaVX, St. Jude Medical, St. Paul, Minnesota) under deep sedation using disoprivan or midazolam. The invasive electrophysiologic study was performed using a recently published standardized protocol and pacing from multiple sites from the right and left ventricle (programmed stimulation and/or burst stimulation). In brief, during sinus rhythm, a left ventricular substrate map (bipolar voltage map) was constructed using a 3.5 cooled-tip ablation catheter (NaviStar Thermo, Cool, EZ-Steer NAV Thermo, Cool, Biosense Webster). The zones of scar (bipolar voltage ≤0.5 mV), and zones of damaged myocardium within the scar border zone (bipolar voltage of 0.5 to 1.5 mV) were identified. Ablation strategies were then based on the individual clinical ventricular arrhythmia and documented structural ventricular abnormalities (scar areas and underlying structural heart disease).
The clinical ventricular arrhythmias were defined as either any spontaneously occurring electrocardiographically documented ventricular arrhythmia, or, if only ICD-Holter data were accessible (without electrocardiographic documentation), induced ventricular arrhythmia with a cycle length comparable to ICD-documented cycle length.
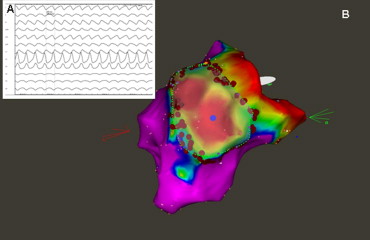
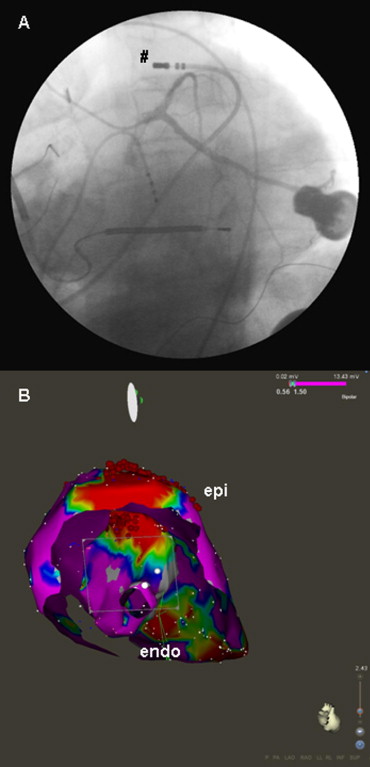
In patients with ischemic cardiomyopathy (ICM) and monomorphic VT ablation was directed towards the border zone of the documented scar region. The site of ablation was chosen according to the congruence of local pace map and the clinical arrhythmia electrocardiogram. Regional (or circumferential) scar encircling ablation was performed, as previously presented ( Figure 1 ). In patients with nonischemic cardiomyopathy (NICM), scar regions were identified, and linear ablation was directed to the interconnect scar and other electrically inert tissue areas (e.g., mitral valve annulus, other scar region, aortic valve annulus; Figure 2 ). The location of the ablation was again chosen according to the congruence between clinical arrhythmia and the local pace map electrocardiogram. In patients with documented ventricular fibrillation (VF), scar mapping was performed, and focal ablation was directed toward areas presenting with Purkinje-like potentials within the scar border zone (producing ventricular premature beats with electrocardiographic morphology comparable to the clinical inductors of VF). If patients had ongoing hemodynamically stable ventricular tachycardia (VT), activation mapping was performed, and ablation was directed toward critical components of the VT. The ablation strategy was individually adapted to the structural abnormalities identified during substrate mapping. In cases in which no ablation success during ablation in the left ventricle was achieved, an epicardial percutaneous ablation approach was used when the electrocardiographic findings of the clinical arrhythmia was susceptible of an epicardial origin ( Figure 3 ). In cases in which clinical ventricular arrhythmia on the electrocardiogram was suspicious of a right ventricular origin (n = 3), mapping and ablation was also directed toward the right ventricle.
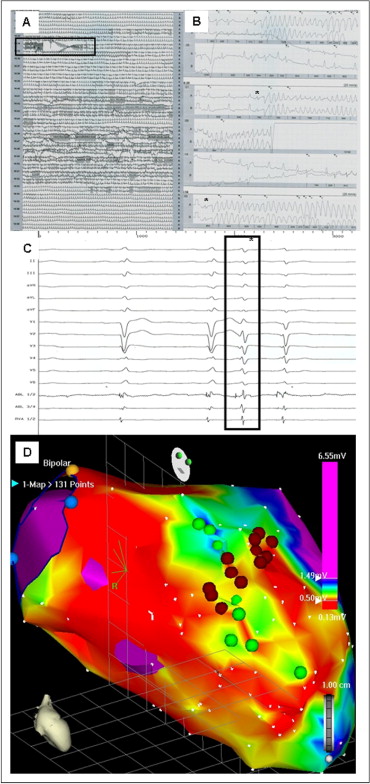
Patients with mechanical devices for left ventricular support were studied in sinus rhythm using a transseptal (intra-aortic balloon pump) or transaortic (extracorporal membrane oxygenation) approach during ongoing device therapy.
During ablation, all inducible ventricular arrhythmias were targeted, but the procedure was stopped if other ventricular arrhythmias after ablation of the clinical needed direct current-shock defibrillation >3 times. The extent of the ablation was left to the discretion of the operator. In all patients, programmed ventricular stimulation to identify procedural success was performed after ablation using ≥2 right ventricular and 2 left ventricular pacing sites (2 pacing cycle length with ≤3 extrastimuli and burst pacing up to a cycle length of 250 ms) if no further arrhythmia was induced. Complete success was considered an inability to induce any sustained ventricular arrhythmia at the end of the procedure. Partial success was defined as the noninducibility of all clinical VTs but the induction of ≥1 nonclinical VT. Failure was considered present if ≥1 clinical VT was inducible at the end of the procedure.
During follow-up, the patients’ antiarrhythmic medication was unchanged from their chronic medication before the onset of ES, except for those patients with ablation failure (for whom the medication was intensified, including treatment with amiodarone plus mexiletine plus β blockade; Table 1 ).
| Characteristic | Value |
|---|---|
| Age (years) | |
| Mean ± SD | 68 ± 10 |
| Range | 33–85 |
| Ejection fraction (%) | |
| Mean ± SD | 28 ± 15 |
| Range | 13–62 |
| Clinical ventricular tachycardia cycle length (ms) ⁎ | |
| Mean ± SD | 397 ± 103 |
| Range | 190–600 |
| Implantable cardioverter defibrillator-treated episodes within 7 days | |
| Mean ± SD | 16.2 ± 11.2 |
| Range | 5–180 |
| Interval from implantable cardioverter defibrillator implant to electrical storm (months) | |
| Mean ± SD | 33 ± 31 |
| Range | 0.5–92 |
| Transferred from collaborating hospitals (n) | 24 (75%) |
| Chronic antiarrhythmic medication | |
| β-Blocker medication | 31 (97%) |
| Class I antiarrhythmic drugs | 6 (19%) |
| Amiodarone | 22 (69%) |
| Previous ventricular tachycardia ablation | 4 (13%) |
| Cardiogenic shock | 8 (25%) |
| Invasive ventilation | 7 (22%) |
| Clinical arrhythmia | |
| Monomorphic ventricular tachycardia | 29 (91%) |
| Polymorphic ventricular tachycardia/ventricular fibrillation | 3 (9%) |
| Transseptal access to left ventricle | 3 (9%) |
| Epicardial approach | 3 (9%) |
| Acute ablation | 5 (16%) |
| Mechanism of clinical arrhythmia | |
| Scar-related | 24 (75%) |
| Bundle branch reentry | 2 (6%) |
| Mitral annulus associated reentry | 3 (9%) |
⁎ Excluding patients with ventricular fibrillation as clinical arrhythmia.
All patients underwent ICD implantation (5 patients with biventricular pacing devices) and were followed up after 1, 3, and 6 months, and every 6 months thereafter. Follow-up included ICD-Holter interrogation and documentation of any ventricular arrhythmia episode triggering ICD treatment. Only appropriate ICD therapies to terminate ventricular arrhythmia were considered. The primary end point was recurrence of sustained ventricular arrhythmia during the follow-up period. The interval to the first appropriate ICD therapy was documented. Any recurrence of ES was documented. Overall rhythm success was defined as no sustained ventricular arrhythmia documented by the ICD or on an electrocardiogram after the first ablation procedure. Cumulated rhythm success was considered success after the initial hospital stay, including multiple ablation sessions.
All procedure-related adverse events were monitored, and death and the cause of death were documented during follow-up.
The patient characteristics are described as the mean ± SD for quantitative data and the number (percentage) for categorical variables. Follow-up is given as the median (range). The recurrence of ventricular arrhythmia episodes during follow-up is given as the percentage of the overall patients treated. The Kaplan-Meier technique was used to calculate the cumulative event rates for survival, freedom from ventricular arrhythmia recurrence (overall rhythm success), and freedom from ventricular arrhythmia recurrence after hospital discharge (cumulated rhythm success). Statistical analyses were performed using the Statistical Package for Social Sciences statistical software, version 18.0 (SPSS, Chicago, Illinois).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


