33 Catheter Ablation of Cardiac Arrhythmias
Energy Sources for Catheter Ablation
Initially, direct current (DC) shocks were delivered through the ablating catheter to achieve destruction of endocardial tissue. However, the effects of DC shock were often traumatic, unpredictable, and patchy. Blood surrounding the catheter tip could vaporize during the procedure and cause marked local injury to the myocardium. Not infrequently, the catheter tip also disintegrated. It then became apparent that radiofrequency (RF) energy, a type of alternating current (AC) already in use for electrocautery, could be modulated and applied through the catheter to create discrete and well-defined lesions. Subsequent experience showed that as long as tissue temperatures did not exceed 100°C, RF energy would not cause barotrauma. Furthermore, RF delivery is relatively painless and can be titrated to achieve the desired degree of tissue damage. Minimal muscle or nerve stimulation also meant that ablations could be performed without general anesthesia. Because of its safety and efficacy (Table 33-1), RF energy has become the preferred and most widely delivered form of energy for arrhythmia ablation.
Table 33-1 Outcomes of Catheter-Delivered Radiofrequency Ablation
| Type of Arrhythmia | Success Rate (%) | Complications (Rate) |
|---|---|---|
| AVNRT | >95 | AV block (1%), pericarditis or cardiac tamponade (0.3%) |
| AVRT | AV block (<1%), cardiac tamponade (0.1% to 1.1%), pericarditis (0.2%), stroke (0.15%), coronary artery dissection (rare) | |
| AV node ablation | 98–100 | Sudden death (rare) |
| Atrial flutter | AV block (rare), stroke (rare) | |
| Focal atrial tachycardia | 86 | AV block, cardiac tamponade, stroke, phrenic nerve damage (collectively 1%–2%) |
| Atrial fibrillation | Stroke (0.1% to 5%), cardiac tamponade (1%), LA flutter (up to 30%), phrenic nerve damage (<0.5%), PV stenosis (uncommon), atrioesophageal fistula (rare) | |
| Idiopathic VT | 85–100 | Cardiac tamponade (Rare) |
| Ischemic VT | 54–81 | MI, stroke, arterial complications, death (1%–2%) |
AF, atrial fibrillation; AP, accessory pathway; AV, atrioventricular; AVRT, atrioventricular reentrant tachycardia; AVNRT, atrioventricular nodal reentrant tachycardia; LA, left atrial; MI, myocardial infarction; PV, pulmonary vein; VT, ventricular tachycardia.
* Reported success rates vary widely, depending on definition of “success,” number of repeat ablations, quality of postoperative surveillance, and so forth.
Radiofrequency Catheter Ablation of Nodal Reentrant Tachycardias
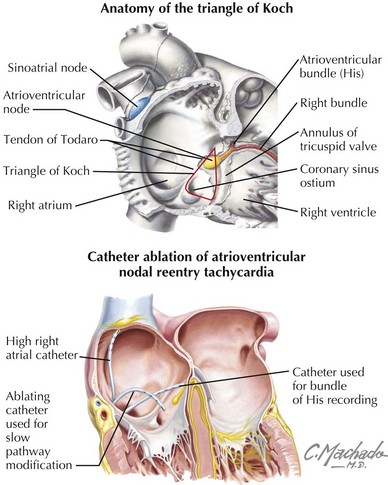
Atrioventricular nodal reentrant tachycardia (AVNRT) is the most common type of paroxysmal SVT (Chapter 27). Although the exact nature of the tachycardia circuit remains uncertain, it is thought that the atrioventricular (AV) node and at least two discrete atrio-nodal tracts of different conduction velocities and refractoriness are involved in this arrhythmia. The most common type of AVNRT is referred to as slow-fast AVNRT. In individuals with slow-fast AVNRT, the “slow” atrio-nodal pathway, which is located in the inferior portion of the triangle of Koch (Fig. 33-1) between the coronary sinus (CS) ostium and tricuspid annulus, forms the antegrade limb of the tachycardia circuit, while the “fast” atrio-nodal pathway—located superior to and behind the tendon of Todaro, level with the apex of the triangle of Koch—conducts retrogradely to the atrium. Other forms of AVNRT have been described, such as fast-slow AVNRT, a type that propagates in a direction opposite to that just mentioned, and a third type that utilizes two slow pathways (slow-slow AVNRT).
The decision to use RF catheter ablation (RFCA) to treat AVNRT is a matter of clinical judgment and patient preference. If the tachycardia occurs frequently or is not well tolerated (either physically or psychologically), or if the patient is disinclined to try antiarrhythmic drugs, then RFCA may be recommended as first-line therapy, particularly given the improvements in RFCA in recent years. Enthusiasm for RFCA was initially limited, because early attempts to break the reentrant circuit by ablating the fast pathway—which lies in close proximity to the compact AV node and His bundle—were accompanied by an unacceptably high incidence of heart block (up to 20%). Following these early studies it was found that ablation or modification of the slow pathway (typically located further away from the AV node and His bundle) was equally effective and much safer. Mapping of the slow pathway is achieved by positioning the catheter within the inferior aspect of the triangle of Koch (see Fig. 33-1) and manipulating it until a delayed, multicomponent atrial potential (thought to represent slow-pathway depolarization) is recorded at the catheter tip. Alternatively, fluoroscopy and anatomic landmarks can be used to localize a specific site where the local ventricular deflection is much larger than the atrial signal. RF energy is then applied to this site. The goal of this overall strategy is to initiate RF ablation at the more distal points in the slow pathway, allowing for subsequent RF applications further superiorly and proximally if the initial therapy is unsuccessful. When necessary, the two methods can be combined to locate more precisely sites amenable to RFCA. When RF energy is applied to the correct site, a transient, accelerated junctional tachycardia is nearly always observed. However, this finding is not specific. Up to 65% of therapeutically ineffective RF applications are also associated with junctional tachycardias. Nonetheless, the absence of a junctional response after 10 to 15 seconds of heating should prompt discontinuation of RF delivery and movement of the ablating catheter to a different location.
For typical AVNRT, slow-pathway modification delivers a cure rate of more than 95% (see Table 33-1). There remains a small but finite risk of inadvertent AV node damage during the procedure, a complication that may require treatment with permanent cardiac pacing.