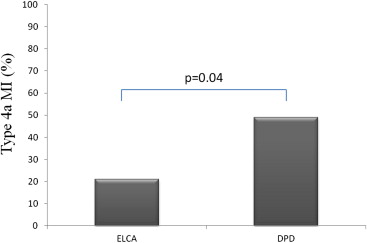
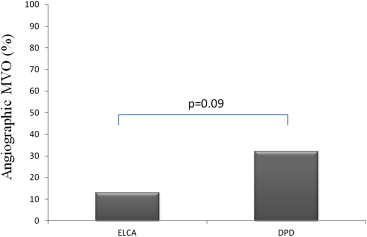
Laser atherectomy might decrease procedural complications during percutaneous coronary intervention (PCI) of degenerated saphenous vein grafts (SVGs) in case of unstable or thrombotic lesions because of its ability to debulk and vaporize thrombus. We aimed at prospectively evaluating the safety and efficacy of excimer laser coronary angioplasty (ELCA) as a primary treatment strategy in consecutively unstable patients undergoing PCI of degenerated SVG lesions. Seventy-one consecutive patients with non–ST elevation acute coronary syndrome (mean age 69 ± 10 years, 66 men [89%]) undergoing PCI of degenerated SVG were enrolled in a prospective case-control registry, using 2 different distal protection devices (DPDs; FilterWire EZ [Boston Scientific, Natick, Massachusetts; n = 24] and SpiderRX [Ev3, Plymouth, Minnesota; n = 23]) or ELCA (n = 24). Primary end points of the study were incidence of angiographic microvascular obstruction (Thrombolysis In Myocardial Infarction flow grade of <3 or Thrombolysis In Myocardial Infraction flow grade of 3 with myocardial blush grade 1 to 2) and incidence of type IVa myocardial infarction. Angiographic microvascular obstruction incidence tended to be less in ELCA-treated patients compared with DPD-treated patients (3 [13%] vs 15 [32%], p = 0.09). Type IVa myocardial infarction incidence was more in DPD-treated patients compared with ELCA-treated patients (23 [49%] vs 5 [21%], p = 0.04). In conclusion, in patients with non–ST elevation acute coronary syndrome undergoing PCI of degenerated SVG, ELCA compared with DPD, is associated with a trend for better myocardial reperfusion and a lesser incidence of periprocedural necrosis. Controlled randomized trials are warranted to confirm these early observations.
Approximately, 1/2 of all saphenous vein grafts (SVGs) may occlude or show critical lesions within 10 years of coronary artery bypass graft surgery. Patients undergoing percutaneous coronary intervention (PCI) of SVG are at risk of distal coronary embolization, with increased risk of periprocedural myocardial infarction (MI), that may lead to an increased mortality at both short- and midterm follow-up. By limiting atheroembolism, the use of embolic distal protection devices (DPDs) has become the standard-of-care during PCI of SVG, and multiple randomized trials have confirmed that this strategy is associated with a reduced rate of adverse events. However, DPD exhibits some limitations, as in case of absence of a proper landing zone, when a stenosis is located close to the distal graft anastomosis, which may occur in nearly up to 50% of SVG PCIs, or in case of acute occlusion of SVG. Moreover, when filters are used, a transient impairment of epicardial flow after stenting, filter “microvascular obstruction (MVO)” has been observed in up to 1/3 of cases. Moreover, in some cases, it is impossible to cross the lesion with DPD or filter apposition is poor because of tortuosity at the site of the landing zone. Finally, in case of unstable lesions, filter crossing of thrombotic lesions may lead to embolization by itself. Current guidelines support the use of embolic protection devices during PCI of SVG when technically feasible (class I, level of evidence B). Of note, previous experiences suggested that laser atherectomy may decrease acute procedural complications during treatment of degenerated SVG, including lesions not amenable to DPDs. Excimer laser coronary angioplasty (ELCA) may debulk underlying plaque and vaporize thrombotic material often present in degenerated SVG, and recent registries have shown a very low complication rate in challenging subsets of patients, thus, ELCA may have a role in PCI of SVG. In this prospective case-control study, we aimed at assessing safety and efficacy of ELCA in patients with non–ST elevation acute coronary syndrome (NSTE-ACS) undergoing SVG PCI compared with the use of DPD.
Material
Ninety-one consecutive patients presenting with NSTE-ACS and undergoing filter-assisted or ELCA-assisted PCI of degenerated SVG were prospectively enrolled from June 2009 to May 2011. DPD included the EPI FilterWire EZ (Boston Scientific, Natick, Massachusetts) and the SpiderRX distal embolic protection system (Ev3, Plymouth, Minnesota). Laser atherectomy was carried out using the CVX-300 Excimer Laser (Spectranetics Corporation, Colorado Springs, Colorado). NSTE-ACS was defined as chest pain at rest in the last 48 hours preceding the admission associated with evidence of transient ST segment depression on 12-lead electrocardiogram and normal (unstable angina) or elevated (non–ST elevation MI) serum troponin T (TnT) levels. Predefined exclusion criteria were multivessel PCI (n = 10 patients), MI within the last 6 months (n = 7 patients), and left ventricular systolic function <30% (n = 3 patients). Thus, 71 patients were included in the analysis. In all patients, cardiovascular risk factors were carefully examined, including family history of early coronary artery disease (first degree relative with a history of MI <60 years), diabetes (fasting blood glucose level >126 mg/dl or treated diabetes), dyslipidemia (levels of low-density lipoprotein >130 mg/dl, high-density lipoprotein <45 mg/dl, triglycerides >150 mg/dl, or total cholesterol >200 mg/dl), smoking, and hypertension (systolic blood pressure >140 mm Hg and/or diastolic blood pressure >90 mm Hg or treated hypertension). Additionally, statin treatment at the time of admission was also reported. Additionally, intracoronary drugs administered at the time of SVG PCI were collected. The study was approved by the local ethics committee, and after a complete explanation of the aims and details of the study, all patients gave their informed consent before entering the study.
Before PCI, all patients received 100 mg of aspirin and oral clopidogrel (with a loading dose of 600 mg if not already on clopidogrel). Intravenous unfractionated heparin was administered to maintain procedural activated clotting time from 200 to 300 seconds. PCI was performed with 6/7/8 Fr access by way of the radial or femoral route. Of note, the femoral or radial approach was selected according to operator preferences when 0.9- or 1.4-mm laser catheters were used; the femoral approach was selected when 1.7- or 2-mm laser catheters were used. When feasible (28 patients [63%]), DPD and ELCA were used without preinflation. Preinflation with a small balloon (1.5 or 2 mm in diameter) was used in 5 patients treated using DPD and in 2 patients treated using ELCA. When ELCA was performed, lasing was started with a delivery rate of 25 Hz and an energy density of 45 mJ/mm 2 and was increased if necessary (maximum energy 80 mJ/mm 2 for the moderately calcified lesion). The laser catheter was moved forward at a speed of 0.2 to 0.5 mm/s. During lasing the “saline flush” technique was applied to facilitate laser-transmitted pressure waves. Laser catheters with concentric tips and size of 0.9, 1.4, 1.7, and 2.0 mm were used depending on the lesion subset and vessel size.
Angiograms were blindly analyzed by 2 experienced operators (GN and FB). Using quantitative coronary angiography (CASS QCA 5.9; Pie Medical Imaging BV, Maastricht, The Netherlands), measurements were made in a single projection showing the most severe stenosis using standard procedure; reference vessel diameter, minimal lumen diameter, and percent diameter stenosis were blindly measured before and after procedure according to previously validated methods. Additionally, total stent length and diameter have been collected. Angiographic analysis included Thrombolysis In Myocardial Infarction (TIMI) flow grade, corrected TIMI frame count (cTFC), myocardial blush grade, thrombus score. Angiographic MVO was defined as TIMI flow grade ≤2 after vessel reopening or TIMI flow grade 3 with a final myocardial blush grade <2. Details about the angiographic analysis are reported in the Supplementary Data .
TnT was measured using Roche Diagnostics reagents on an Elecsys 2010 immunoassay analyzer (F. Hoffmann-La Roche Ltd., Basel, Switzerland). The criterion of coefficient of variation ≤10% in our laboratory was met by a TnT concentration of ≥0.03 ng/ml. TnT was analyzed at admission, before PCI, and at 12 and 24 hours after PCI. According to the European Society of Cardiology guidelines, type IVa MI was defined by elevation of TnT values >5 × ninety-ninth percentile upper reference limit in patients with normal baseline values (less than or equal to the ninety-ninth percentile upper reference limit) or an increase of TnT values >20% if the baseline values are elevated and are stable or decreasing. In addition, either (1) symptoms suggestive of myocardial ischemia, or (2) new ischemic electrocardiographic changes or new left bundle branch block, or (3) angiographic loss of patency of a major coronary artery or a side branch or persistent slow or no flow or embolization, or (4) imaging demonstration of new loss of viable myocardium or new regional wall motion abnormality were required. Primary study end points were (1) the incidence of angiographic MVO and (2) the incidence of type IVa MI. Secondary end points were levels of creatinine kinase (CK)-MB and TnT 24 hours after the procedure.
Continuous variables are presented as mean ± SD, whereas dichotomous variables are reported as percentages. Comparisons between groups were carried out by Student t test and by chi-square test, as appropriate, whereas analysis of variance was performed for multiple comparisons followed by Bonferroni adjustment. Statistical analyses were performed using SPSS 17.0 software (SPSS Inc., Chicago, Illinois) and statistical significance was attributed to p values <0.05.
Results
Baseline clinical, enzymatic, angiographic, and procedural data were similar in the 2 treatment groups ( Tables 1 and 2 ). DPD used during PCI were the FilterWire EZ in 24 (34%) and the SpiderRX distal embolic protection system in 23 patients (32%). ELCA was used in 24 patients (34%). Of note, ELCA was used for SVG lesions in which the filter could not be positioned because of distal localization of the stenosis (n = 4 patients), marked tortuosity of the graft (n = 6 patients), or completely occluded graft (n = 4 patients), whereas in the remaining cases ELCA use was left at operators’ choice. Of note, statin use at admission was similar between the 2 groups, whereas intracoronary nitrates were administered in all patients but 5 (2 in the ELCA-treated group and 3 in the DPD-treated group) and intracoronary adenosine was administered in 3 patients only (1 in the ELCA-treated group and 2 in the DPD-treated group).
| Variable | ELCA, n = 24 (%) | DPD, n = 47 (%) | p |
|---|---|---|---|
| Age (yrs) | 71 ± 10 | 68 ± 10 | 0.34 |
| Men | 17 (71) | 37 (79) | 0.56 |
| Smoker | 11 (46) | 17 (36) | 0.45 |
| Family history of coronary artery disease | 16 (67) | 25 (53) | 0.32 |
| Arterial hypertension ∗ | 12 (50) | 25 (53) | 0.81 |
| Dyslipidemia † | 16 (67) | 29 (62) | 0.79 |
| Diabetes mellitus | 12 (50) | 25 (53) | 0.81 |
| Previous MI | 10 (42) | 20 (43) | 0.99 |
| Unstable angina pectoris | 8 (33) | 14 (30) | 0.79 |
| Statin use | 22 (92) | 43 (91) | 0.97 |
| Basal CK (IU/L) | 98.54 ± 35.39 | 120.08 ± 45.59 | 0.56 |
| Basal CK-MB (ng/ml) | 3.60 ± 4.18 | 3.72 ± 4.48 | 0.91 |
| Basal TnT (ng/ml) | 0.13 ± 0.09 | 0.13 ± 0.10 | 0.78 |
| Native vessel involved | |||
| Left anterior descending | 7 (29) | 13 (28) | |
| Left circumflex | 10 (42) | 22 (47) | 0.91 |
| Right coronary | 7 (29) | 12 (25) | |
| Graft segment | |||
| Ostial-proximal | 10 (42) | 17 (36) | |
| Mid | 8 (33) | 21 (45) | 0.98 |
| Distal anastomosis | 6 (25) | 9 (19) | |
| Graft age (yrs) | 8.9 ± 4.1 | 9.2 ± 3.7 | 0.84 |
| Degeneration score | 1.8 ± 0.9 | 2.0 ± 0.8 | 0.57 |
| Lesion length (mm) | 25.43 ± 12.62 | 28.58 ± 18.54 | 0.40 |
| Number of stents | 1.7 ± 1.4 | 1.8 ± 1.6 | 0.68 |
| Total stent length (mm) | 29.21 ± 14.5 | 33.2 ± 21.2 | 0.32 |
| Total stent diameter (mm) | 3.1 ± 0.6 | 3.2 ± 0.7 | 0.68 |
| Reference vessel diameter before procedure (mm) | 3.2 ± 0.7 | 3.3 ± 0.8 | 0.73 |
| Minimal lumen diameter before procedure (mm) | 0.9 ± 0.5 | 0.9 ± 0.6 | 0.89 |
| Diameter stenosis before procedure (%) | 82 ± 11 | 81 ± 9 | 0.84 |
| Reference vessel diameter after procedure (mm) | 3.5 ± 0.8 | 3.6 ± 0.8 | 0.86 |
| Minimal lumen diameter after procedure (mm) | 3.3 ± 0.7 | 3.4 ± 0.7 | 0.87 |
| Diameter stenosis after procedure (%) | 12 ± 3 | 11 ± 3 | 0.89 |
| Predilatation | 10 (42) | 14 (58) | 0.80 |
| Drug-eluting stent use | 20 (83) | 35 (75) | 0.55 |
| Postdilatation | 11 (46) | 23 (49) | 0.98 |
| Basal TIMI flow grade | |||
| 1 | 5 (42) | 7 (58) | |
| 2 | 10 (31) | 22 (69) | 0.70 |
| 3 | 9 (33) | 18 (67) | |
| Basal MBG | |||
| 1 | 5 (21) | 13 (18) | |
| 2 | 9 (37) | 15 (32) | 0.69 |
| 3 | 10 (42) | 19 (40) | |
| Basal cTFC | 46.41 ± 31.69 | 45.31 ± 31.42 | 0.89 |
∗ Arterial hypertension: systolic blood pressure >140 mm Hg and/or diastolic blood pressure >90 mm Hg or treated hypertension.
† Dyslipidemia: low-density lipoprotein >130 mg/dl, high-density lipoprotein <45 mg/dl, triglycerides >150 mg/dl, or total cholesterol >200 mg/dl.
| Variable | ELCA, n = 24 (%) | DPD, n = 47 (%) | p |
|---|---|---|---|
| Procedural Safety | |||
| Intraprocedural death | 0 | 0 | 0 |
| Emergency coronary bypass surgery | 0 | 0 | 0 |
| Perforation | 0 | 0 | 0 |
| Type C dissection or worse | 1 (4) | 0 | 0.90 |
| Acute closure | 0 | 0 | 0 |
| Angiographic analysis | |||
| TIMI flow grade 3 | 22 (92) | 38 (81) | 0.20 |
| cTFC after ELCA | 23.04 ± 9.60 | — | — |
| Final cTFC | 16.33 ± 5.36 | 33.93 ± 22.65 | 0.001 |
| MBG 2 to 3 | 21 (88) | 32 (68) | 0.09 |
| Angiographic MVO | 3 (13) | 15 (32) | 0.09 |
| Angiographic evidence of distal embolization | 1 (4) | 2 (4) | 0.98 |
| Periprocedural MI | |||
| Type IVa MI | 5 (21) | 23 (49) | 0.04 |
| CK 24 h after procedure (IU/L) | 73.19 ± 56.12 | 190.85 ± 140.57 | 0.001 |
| CK-MB 24 h after procedure (ng/ml) | 9.44 ± 5.02 | 16.84 ± 5.50 | 0.001 |
| TnT 24 h after procedure (ng/ml) | 0.51 ± 0.40 | 1.48 ± 0.78 | 0.001 |
Angiographic MVO incidence tended to be less in ELCA-treated patients compared with DPD-treated patients (3 [13%] vs 15 [32%], p = 0.09; Figure 1 ). Final TIMI 3 flow rate was similar between ELCA-treated and DPD-treated patients (22 [92%] vs 38 [81%], p = 0.20), whereas final myocardial blush grade of 2 to 3 rate was tended to be more in ELCA-treated patients compared with DPD-treated patients (21 [88%] vs 32 [68%], p = 0.09). Moreover, cTFC decreased from 46.41 ± 31.69 to 16.33 ± 5.36 (p = 0.01) in the ELCA groups (p = 0.02), whereas cTFC decreased from 45.31 ± 31.42 to 33.93 ± 22.65 (p = 0.17) in the DPD groups (p = 0.13). ELCA use was associated with a significant lower final cTFC compared DPD-treated patients (16.33 ± 5.36 vs 33.93 ± 22.65, p = 0.001).
Type IVa MI incidence was more in DPD-treated patients compared with ELCA-treated patients (23 [49%] vs 5 [21%], p = 0.04; Table 2 and Figure 2 ). TnT levels, as assessed 24 hours after the procedure, were significantly low in ELCA-treated patients compared with DPD-treated patients (0.51 ± 0.40 ng/ml vs 1.48 ± 0.78 ng/ml, p = 0.001; Table 2 and Figure 3 ). CK-MB levels, as assessed 24 hours after the procedure, were significantly low in ELCA-treated patients compared with DPD-treated patients (9.44 ± 5.02 ng/ml vs 16.84 ± 5.50 ng/ml, p = 0.001; Table 2 and Figure 3 ).