Carotid Artery Trauma
MOHAMED F. OSMAN and JONATHAN D. GATES
Presentation
A 51-year-old man presents to the emergency department with a stab wound sustained 12 hours ago to the right side of the neck. He describes a tender mass around the area. He admits to mild clumsiness and numbness in his left hand and left leg, but denies difficulty with breathing or swallowing, vocal changes, hematemesis, hemoptysis, or other neurologic symptoms. Examination of the neck reveals multiple small wounds and a 2-cm stab wound to zone II (between the cricoid cartilage and the angle of the mandible) of the right neck just anterior to the sternocleidomastoid muscle (SCM) with a moderate-sized firm pulsatile hematoma (Fig. 1). There is no active bleeding, air bubbling from the wound, or crepitus in the neck. He is in no respiratory distress or stridor and has no palpable thrill or bruit on auscultation of the neck. The patient is hemodynamically stable and has no wounds in other regions of the body. Neurologic examination reveals a mild left pronator drift and a mild decrease to light touch sensation in the left arm and left leg.
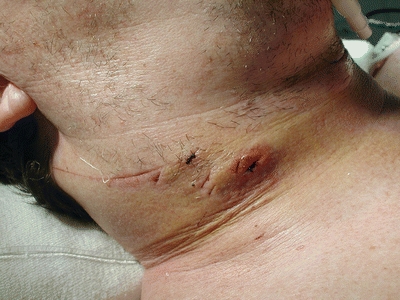
Figure 1 Stab wound to zone II of the neck.
Differential Diagnosis
This man has suffered a single stab wound to the neck with a moderate-sized hematoma. A focused and detailed clinical evaluation reliably identifies patients with vascular injuries who require treatment. A negative physical examination with observation has a negative predictive value of 90% to 100% for vascular injuries. In the event that there are no obvious clinical signs of vascular or aerodigestive tract injury, it is essential to determine the depth of this wound through local exploration. If the wound is small, it must be explored with the use of local anesthesia to enlarge the wound and determine whether or not it has penetrated the platysma. If the wound is large and gaping, often elevation of the tissue is sufficient to determine that the platysma has been violated. Wounds that are superficial to the platysma are treated with debridement and closure while deeper wounds classify as penetrating trauma and raise the suspicion for deeper injury to vital structures in the neck.
This patient has soft signs of vascular injury, namely a stable neck hematoma in the setting of an injury tract in the vicinity of major vessels. Other soft signs include history of bleeding at the scene, cranial nerve injury, and unequal upper extremity blood pressure measurements. Soft signs allow time for further investigation with selective operative exploration as an option. Hard signs include refractory hypotension, pulsatile bleeding, bruit or thrill, expanding hematoma, and loss of pulse with stable or evolving neurologic deficit. Hard signs mandate urgent exploration. Ninety-seven percent of patients with hard signs have a vascular injury, as opposed to only 3% of those with soft signs.
Early airway evaluation and a low threshold for airway control are paramount in the initial evaluation of these patients with significant neck wounds. There is no evidence for airway compromise in this case. Obvious signs of airway injury (respiratory distress, hemoptysis, stridor, or air bubbling from the wound) are indications for operative exploration and repair. There is no obvious sign of esophageal or pharyngeal injury as there is no air bubbling from the wound during the act of swallowing and no reported odynophagia. Clinically, he has evidence of a mild right hemispheric stroke. At the top of the differential, one must consider penetrating injury to the carotid artery with an acute mild stroke from embolic debris. Spinal cord, cranial nerves (IX, X, XI, and XII), and brachial plexus injuries are other possibilities but are inconsistent with this physical exam.
Workup
Given that the patient is hemodynamically stable, the wound has penetrated deep to the platysma, and soft signs of vascular injury are present, further workup is essential and appropriate. Preoperative CT scan of the head is essential in this stable patient to delineate the presence or absence of suspected stroke and determine whether or not there is a hemorrhagic component. Computerized tomographic angiography (CTA) has become an invaluable screening tool for arterial injuries in some locations, the neck being one of them. In the setting of penetrating cervical injuries, CTA has a 90% sensitivity and 100% specificity for vascular injuries that require treatment. This study modality, CTA, may be limited in the setting of metallic foreign bodies such as bullet or missile fragments (especially shotgun injuries) obscuring the cervical vasculature through artifact. Arteriography taken in various planes allows the radiographer to vary the relationship of the stationary fragment to the underlying vasculature without artifactual degradation of the image. Angiography supplies additional information and is perhaps more sensitive with respect to minor intimal injury. Angiography was at one point the diagnostic study of choice before the availability and speed of the current generation of CT scan machines. Arteriography has become a second-line diagnostic study in the presence of artifact but also has the distinct advantage that it offers therapeutic endovascular interventional options at the same time. CTA is much more widely employed in these scenarios due to its ease of availability and plethora of useful information.
Ultrasonography has become the standard screening tool for carotid disease in the elective evaluation of suspected atherosclerotic disease. It has been used for penetrating neck trauma, but its utility is limited to zone II neck injuries. In addition, subcutaneous air, fragments, and hematomas make ultrasound less reliable. Magnetic resonance angiography (MRA) may aid in the diagnosis of arterial injury but requires the patient be moved to the magnetic resonance suit, rendering the patient less available for ongoing care and perhaps at risk for movement of any metallic foreign body subjected to the magnetic field.
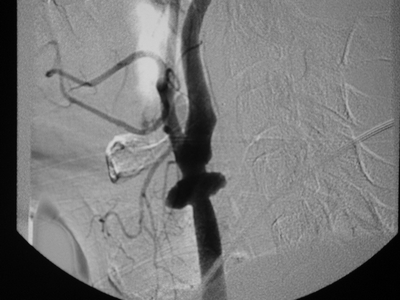
In this case, CT scan of the head without intravenous contrast revealed a 3-cm ischemic, nonhemorrhagic stroke in the right frontal lobe anterior to the motor strip (Fig. 2). The neck CT with intravenous contrast showed an extraluminal collection of contrast at the distal common carotid artery consistent with a pseudoaneurysm with preservation of antegrade flow (Figs. 3 and 4). An angiogram was performed with an option for an endovascular solution; however, the potential contamination of the neck wound from aerodigestive content was a concern (Fig. 5).
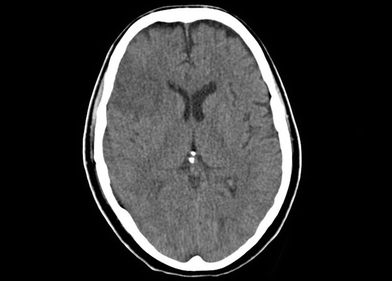
Figure 2 CT scan of the head shows a bland stroke in the frontal lobe.
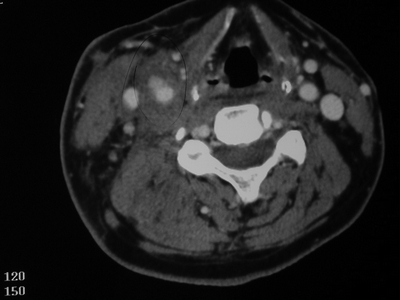
Figure 3 CTA of the neck reveals a right common carotid pseudoaneurysm.
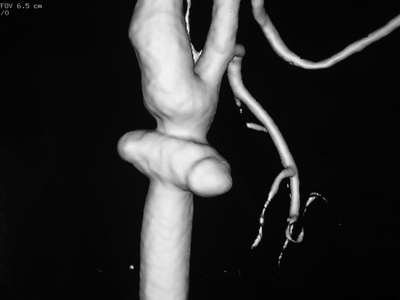
Figure 4 Three-dimensional reconstruction of the CTA of the neck demonstrating the pseudoaneurysm.
Diagnosis and Treatment
This patient has penetrating trauma to the right common carotid artery with localized pseudoaneurysm formation. The arterial wall has been disrupted, and the leaking arterial blood is contained by surrounding hematoma and periarterial tissues. This resulted in a moderate-sized bland infarct of the right frontal lobe. A small thrombus or more likely a platelet plug must have formed at the site of the injury or from turbulence within the pseudoaneurysm and embolized to the brain causing an ischemic stroke. The carotid lesion also may rupture, given the tenuous nature of the wall of the pseudoaneurysm. Clinically, the stroke is relatively small. Radiologically, it is of moderate size and hemorrhagic transformation with revascularization is of concern.
Catheter-based endovascular interventions in trauma have evolved from the early embolization of end vessels, to stent placement, and currently covered stents for larger defects in the wall of arteries. For zones I and III injuries, endovascular exclusion of a pseudoaneurysm, partial transection, or arteriovenous fistula remain viable options. Self-expanding covered stents can be safely delivered to these locations with limited morbidity. The anatomically inherent difficulty of attaining good open surgical exposure and control (proximal control in zone I and distal control in zone III) in these injuries makes the endoluminal approach an attractive option. This approach may avoid the morbidity of a median sternotomy, high thoracic incision, or difficult dissection at the base of the skull. Another advantage is that endoluminal therapy can be performed with the patient under local anesthesia, allowing direct assessment of the patient’s neurologic status.
Zone II vascular injuries should be treated by operative repair. Moreover, the possible need for anticoagulation and dual antiplatelet therapy during and after the procedure may represent a risk of transformation of the bland infarct into hemorrhagic infarct. The traumatic nature of the wound, late presentation, and potential aerodigestive tract injury raise the issue of foreign body and infection risk should a covered stent be deployed. If the wound were to become infected, the presence of a covered stent may complicate the situation. Surgical options include exploration and repair of the arterial injury with intraoperative intravenous heparin and secondary prophylaxis with aspirin perioperatively. The risk of intracranial bleeding would be less, and this approach would allow definitive evaluation of the airway and digestive tract as well as allow the option of definitive wound care and removal of a foreign body if present. One must continue with the evaluation of the aerodigestive tracts using bronchoscopy and either endoscopy or water-soluble contrast study.
Special consideration should be given to patients who present with coma, dense hemispheric stroke or documented carotid thrombosis. Currently, the only preoperative outcome predictive marker is the injury-to-revascularization time duration. Early revascularization in this group has consistently demonstrated improvement or stabilization of neurologic function. Late revascularization (more than 24 hours from the time of injury) exposes the patient to the risk of developing reperfusion injury, cerebral edema, uncal herniation, and death. Minor carotid injuries, intimal defects, or small pseudoaneurysm in selected neurologically stable patient can be safely managed nonoperatively.
Surgical Approach
Electroencephalography (EEG) monitors are placed on the head to monitor brain function while the patient is under general anesthesia of the case. If EEG monitoring is not available, then an intravascular shunt may be used, or back pressure may be measured once the clamp is placed on the common carotid artery. If the injury and the repair involve only the common carotid artery, then the overwhelming majority of the time there should be no ipsilateral cerebral ischemia, but it is not impossible as the collateral circulation is an unknown. The patient is kept supine and in a slight Trendelenburg position with the neck carefully positioned for maximal exposure and the head mildly rotated away from the side of injury. The patient is prepped from the chin down to the bilateral knees in anticipation of the need for a thoracic incision or saphenous vein harvest. In vascular trauma, one must anticipate the unexpected and be prepared for proximal and distal control of the common carotid and internal/external carotid arteries, respectively, so as to minimize hemorrhage and time of potential neurologic ischemia prior to addressing the pseudoaneurysm (Fig. 6).
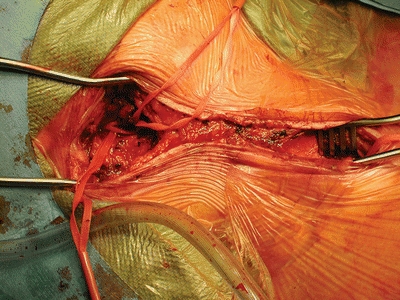
Figure 6 Operative exposure starting with proximal and distal control.