Chapter 99
Carotid Artery Disease
Decision Making Including Medical Therapy
John J. Ricotta, Joseph J. Ricotta
Based on a chapter in the seventh edition by Timothy F. Kresowik and Harold P. Adams, Jr.
Much literature, including multiple prospective randomized trials, have compared carotid endarterectomy (CEA) with “best” medical therapy (BMT), and then subsequently compared CEA with carotid artery stenting (CAS). Despite long experience with carotid bifurcation intervention and a large body of level one evidence addressing its proper application, there is still disagreement about the indications for carotid intervention to prevent stroke. This chapter focuses on evidence-based clinical decision making in patients with lesions of the cervical carotid artery that present as an increased stroke risk, in a variety of specific clinical circumstances. Topics covered include identification of stroke prone conditions, role of medical therapy in stroke prevention, and the current role of interventional treatment for patients with extracranial carotid stenosis. The treatment of the great vessels (see Chapter 105), vertebral arteries (see Chapter 107), acute stroke (see Chapter 97), and interventional treatment techniques (see Chapters 100 and 101) are discussed elsewhere.
The pathophysiology of stroke in patients with carotid bifurcation plaque and the concept of “vulnerable plaque,” are discussed in detail in Chapter 97. Identification of vulnerable plaque is critical to clinical decision making, because these lesions are both targets for maximal medical therapy and are most likely to derive additional benefit from intervention. However, the most precise identification of vulnerable plaque, as described in the literature, relies on several specialized techniques that, although currently the focus of active investigation, are not used in routine patient assessment. As a matter of clinical practice, determination of percent stenosis is the most common method to assess plaque risk, and in general, works quite well.
The various imaging techniques to characterize cerebrovascular disease are discussed in Chapter 98. One of the most important steps in clinical evaluation is the identification and accurate quantification of carotid bifurcation disease, expressed as the degree of stenosis. In a neurologically asymptomatic patient, presence of “significant” carotid bifurcation disease, as defined below, should prompt considering the patient for carotid intervention, whereas absence of such significant disease interdicts consideration of carotid intervention and prompts aggressive medical therapy. In a neurologically symptomatic patient, the presence of significant carotid bifurcation disease is a target for intervention, whereas its absence should prompt a search for another source for symptoms.
To determine the best clinical management of patients with extracranial carotid stenosis, there are two critical questions that must be addressed: (1) What is the risk of stroke related to the carotid bifurcation lesion? (2) What are the intrinsic risks of intervention in a particular patient?
Each of these questions has a number of qualifiers that are discussed in detail; however, if these two essential questions can be addressed, the appropriate management can be selected.
Determining Stroke Risk of A Carotid Lesion
Symptom Status
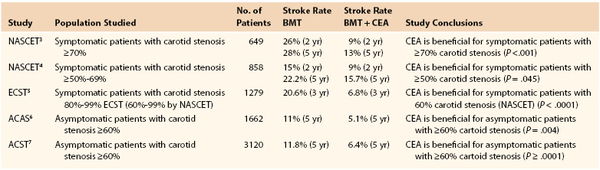
The most important indicator of future stroke risk is the presence of recent (within 6 months) ipsilateral neurologic symptoms. Such plaques are already clinically “vulnerable” or “embologenic.” The stroke risk in the medical arms of two major symptomatic carotid trials1–3 was much higher than that seen in the medical arms of two major asymptomatic trials,4,5 as shown in Table 99-1. Further, the North American Symptomatic Carotid Endarterectomy Trial (NASCET) and European Carotid Surgery Trial (ECST) both demonstrated that stroke risk is highest within the first month after an initial neurologic event and drops off to approach that of an “asymptomatic” lesion by approximately 6 months.6 The observed reduction in stroke risk as the initial event becomes more remote likely reflects “stabilization” or healing of the plaque over time, either spontaneously or in response to medical therapy.
In symptomatic patients with high grade (70%-99%) stenosis, the relationship of the stenosis to future stroke risk is so strong that a causal relationship can be assumed. However, in symptomatic patients with more moderate degrees of stenosis (50%-69%), the benefit of carotid intervention is not as great, implying that the relationship between the bifurcation lesion and stroke is not as clear, and therefore, other potential sources of neurologic symptoms should be excluded before carotid intervention is undertaken. This includes a search for a cardiac source, interrogation of the intrathoracic and intracranial circulation, and brain imaging to evaluate both the presence of ipsilateral infarction (supporting carotid bifurcation intervention) and the existence of intracranial lesions (interdicting carotid bifurcation intervention).
Although ipsilateral neurologic symptoms are the strongest predictors of stroke risk, history of stroke of any kind is also associated with increased risk of subsequent stroke. In a large contemporary natural history study, the Asymptomatic Carotid Stenosis and the Risk of Stroke Study (ACSRS), any previous stroke or a previous contralateral transient ischemic attack (TIA) was an independent predictor of stroke risk in a patient with asymptomatic carotid stenosis.7 The reasons for this are unclear; these patients may represent a group with more aggressive atherosclerosis, or they may have intracranial lesions that compromise the ability to compensate for a subsequent event. Many years ago, Imparato8 observed that an individual patient tends to have the same type of plaque in both carotid arteries. This implies that patients with previous symptomatic carotid plaque are more likely to have vulnerable plaque in the contralateral artery. Whatever the explanation, it is clear that a totally asymptomatic patient and a patient with any previous neurologic events are not at equal risk of stroke.
Degree of Stenosis
In the major symptomatic trials, as well as in natural history studies,1–7 the degree of stenosis was directly related to stroke risk. In NASCET, each decile of stenosis was correlated with an increase in stroke risk, and lesions from 70% to 99% were much more likely to progress to stroke on medical therapy than were lesions of 50% to 69% diameter stenosis, whereas lesions of less than 50% stenosis were unlikely to result in subsequent neurologic symptoms.1,2 This finding of increasing stroke risk with increasing diameter stenosis was also demonstrated in the ECST trial.3 In asymptomatic patients, no linear correlation between diameter stenosis and increased stroke risk was seen in the medical arms of the Asymptomatic Carotid Atherosclerosis Study (ACAS) trial4 or Asymptomatic Carotid Surgery Trial (ACST).5 There are a number of potential explanations for this seeming inconsistency. First, the use of duplex ultrasound (DUS) for diagnosis may not have permitted as accurate a distinction between degrees of stenosis as was possible in ECST and NASCET; these trials quantified stenosis angiographically. Second, the low number of events in the asymptomatic trials may have resulted in inadequate sample size to demonstrate a difference between 60% and 79% and 80% and 99% stenosis. In the subgroup analysis of the ACST study, the mean diameter reduction of the more than 60% stenosis group was 69%, suggesting that the lesions in the trial were not evenly distributed across deciles of stenosis, but rather were weighted toward the “60% to 79%” category.5
Data on plaque character were not recorded in the asymptomatic trials. However, in ASCRS trial, 17% of patients had carotid plaques of “type 4 or 5” (primarily fibrous or homogeneous)12 that were associated with an annual stroke risk of 0.7%, despite greater than 60% diameter stenosis.7 It is reasonable to assume a similar number of “benign” plaques were present in ACAS and ACST. Taken together, this may have resulted in insufficient numbers of truly high risk lesions in the medical arm of the asymptomatic studies to show gradation in risk between deciles of stenosis. Natural history studies of asymptomatic plaques with stenosis ranging from minimal to severe confirmed the increased risk of stroke associated with plaques with more severe stenosis.9–11 Nicolaides et al12 studied the natural history of asymptomatic carotid stenosis in 1121 asymptomatic patients who did not undergo carotid endarterectomy. In these patients, the carotid plaques were carefully measured by standardized and routine ultrasound techniques, analyzed in a core laboratory. Multiple clinical and anatomic characteristics, including degree of stenosis, plaque surface irregularity, and echolucency were recorded. Plaque diameter stenosis was calculated using the “ECST” method, which compared the diameter of the lumen with that of the entire artery at the point of maximal stenosis. Using this methodology, degree of stenosis proved to be a predictor of future stroke risk after multivariate analysis. It is likely that the rigorous standardization of duplex imaging and use of a core laboratory to evaluate images accounts for the differences between the ASCRS study and ACAS or ACST. It is of interest to note that analysis of asymptomatic contralateral stenoses in the NASCET study did find a correlation between degree of stenosis and stroke risk.13 The total 5-year stroke risk in the contralateral artery was 8.0% in patients with contralateral stenosis of less than 60% and 16.2% in those with contralateral 60% to 99% stenosis. The risk of “large artery” ipsilateral stroke for an asymptomatic 60% to 99% stenosis was 9.9% in addition to a 6.0% risk of lacunar stroke (many of which are now thought to result from artery to artery emboli). The overall 5-year risk of stroke was higher in the 75% to 94% stenosis category (18.5%) than it was in the 60% to 74% (14.8%) or the 95% to 99% (14.7%) groups. Although these patients were not identical to neurologically asymptomatic patients, these findings confirmed the relationship of angiographically determined stenosis and increased late stroke risk.
Because the diameter of most carotid arteries fall within the range of a few millimeters, diameter stenosis is a surrogate measure of plaque size or volume. Plaque size has been correlated with plaque composition; larger plaques are less likely to be fibrotic and more likely to contain lipid, intraplaque hemorrhage with a necrotic core, and inflammatory cells.14,15 It is likely then that diameter stenosis is a surrogate measure for plaque size, and in balance, larger plaques are more often ulcerated and contain lipid or intramural hemorrhage. It is clear then that although stenosis is an important feature used to assess stroke risk, on a hierarchal basis, it is not as important as the presence or absence of symptoms.
Plaque Progression
Plaque progression has also been found to be associated with increased risk of subsequent stroke. The preceding natural history studies demonstrated that plaques that progressed from less than 50% to more than 50% stenosis while under observation were at increased risk of stroke, and this was even more marked when the progression was more than 80% stenosis.16,17 Bertges et al,16 studied 1701 asymptomatic carotid arteries over a 9-year period and demonstrated that plaque progression increased the odds risk of subsequent stroke or TIA by 1.68 (P < .001). They found a 9.3% annualized risk of progression, influenced by the degree of initial carotid stenosis and blood pressure. More recently, Sabeti et al17 followed 1065 asymptomatic patients with carotid plaque for a mean of 3.2 years. They documented major adverse cardiovascular events (MACEs) in 40% of patients, progression of carotid diseases in 9.3%, and a correlation between carotid plaque progression and MACEs (odds ratio [OR], 2.01; P < .001), stroke (OR, 2.0: P > .035), and cardiovascular death (OR, 1.75; P = .039).17 It is likely that plaque progression is a manifestation of dynamic plaque events such as inflammation, intraplaque hemorrhage, and/or rupture of the fibrous cap and consequent exposure of necrotic plaque elements to the flow lumen. Thus, patients with plaque progression are at particular risk for subsequent cardiovascular risks, including stroke.
Presence of Contralateral Disease
The presence of contralateral carotid occlusion (CCO) may be associated with increased stroke risk in patients with significant carotid stenosis. Although a post hoc analysis of patients in the ACAS study with CCO18 demonstrated an decreased risk of stroke (3.5% at 5 years) in 168 patients from the medical arm of the trial, the explanation for this is unclear and has not been substantiated by other studies, including ACST.5 AbuRahma et al19 followed 82 patients with more than 60% stenosis and CCO and documented a combined neurologic event rate of 60%, including a stroke rate of 33% at a mean 5-year follow-up, with events distributed between ipsilateral and contralateral arteries. It is likely that the degree of intracranial collateral circulation supplied by the contralateral carotid and vertebral arteries is related to late stroke risk. The adequacy of collateral cerebral circulation can be measured by imaging collateral intracranial circulation or functional assessment of collateral flow by measuring cerebrovascular vasomotor reactivity. Several studies employing this technique have shown that patients with CCO and impaired collateral circulation are at an increased risk of late stroke, whereas CCO patients with relatively normal intracranial hemodynamics have a lower risk of late stroke.20,21
Plaque Character
As noted previously (see Chapter 97), features associated with vulnerable plaque include plaque heterogeneity with increased echolucency, evidence of surface irregularity as manifested by ulcerations on angiography, a thin or disrupted fibrous cap with an adjacent echolucent plaque area, and increased inflammatory infiltrate as seen on magnetic resonance imaging (MRI) or a positron emission tomographic (PET) scan. Moore et al22 first described the relationship between surface irregularity (ulceration) and stroke risk more than 3 decades ago. Asymptomatic lesions that were ulcerated had an annual stroke risk of up to 12%. Fisher et al,23 who studied plaques from both the NASCET and ACS trials, found that ulceration was more common in symptomatic patients, as was the presence of thrombus. The Oxford Plaque study confirmed an association between ulceration and histologic plaque rupture, large lipid core, macrophage infiltration, and general evidence of inflammation.24 More recent studies of plaque from patients with remote symptoms suggested they have a higher incidence of unstable plaque characteristics than do patients who have never been symptomatic.25 Many authors correlated echolucent areas of plaque seen by DUS with symptomatic potential, and this was quantified by Nicolaides et al,26 who used a normalized grey scale to quantify echolucency. Nicolaides et al26 also used normalized DUS to correlate plaque echolucency with the frequency of ipsilateral neurologic symptoms.12,26 Plaque echogenicity was also correlated with the frequency of recurrent ischemic events in symptomatic patients27 and the amount of embolic material recovered after carotid stenting.28 The most comprehensive data on identifying “stroke prone” patients with asymptomatic carotid stenosis was from the ACSRS study.7 This study followed 1121 neurologically asymptomatic patients with known carotid plaque between 50% and 99% stenosis found on ultrasound. By using a combination of clinical and anatomic risk factors, the investigators were able to identify patients at very low (<5%), low (5%-9.9%), moderate (10%-19.9%), and high (>20%) 5-year risk of stroke. The risk factors they found associated with increased risk of stroke for patients with asymptomatic carotid stenosis included percentage of stenosis, smoking history, renal insufficiency, previous contralateral events, plaque area, and plaque character (grey scale median and presence of “discrete white areas” within the plaque).
Evidence of Clinically Silent Emboli
Clinically silent cerebral infarction can manifest in one of two ways: (1) evidence of previous ipsilateral cerebral infarction on computed tomography (CT) or MRI imaging of the brain, or (2) detection of embolic material in the ipsilateral middle cerebral artery using transcranial Doppler (TCD) techniques. In either case, the purpose is to identify emboli that originate from a carotid bifurcation stenosis but do not result in clinically detectable neurologic symptoms.
Routine CT scans taken before operation in asymptomatic patients demonstrated an incidence of silent cortical infarction in up to 20% of cases.29 Subsequent studies correlated the presence of such infarcts with increased risk of subsequent ipsilateral hemispheric symptoms.14,30 A systematic review of 19 studies of TIA patients demonstrated that evidence of acute ischemic lesions on diffusion weighted MRI was associated with an early increased risk of ischemic stroke.31 In addition, a prospective cohort study of asymptomatic individuals correlated the presence of microembolic “hits” detected by TCD with increased risk of subsequent clinical stroke.32 A review of 58 articles investigating the impact of TCD microemboli on subsequent neurologic events was recently reported by Jayasooriya et al.33 The duration of TCD monitoring ranged from 30 minutes to 8 hours, with the most common intervals being 30 or 60 minutes. Several of the studies used multiple monitoring sessions. The prevalence of microemboli was higher in patients with neurologic symptoms. In the asymptomatic patients, between 4% and 30% of patients had microemboli, with a frequency of microemboli of 0.35 to 4 embolic signal events per hour. They found a positive correlation between an increased rate of subsequent stroke or TIA in patients with silent microemboli detected by TCD compared with patients in whom no microemboli were identified (28% versus 2%; P = .001). The authors concluded that there were level one data to support using TCD evidence of microembolization as a risk stratification tool, and that it might be used with other techniques, such as sophisticated carotid plaque imaging to identify increased stroke risk associated with asymptomatic carotid stenosis. This technique is both patient- and operator-dependent. Currently, these factors, along with events in asymptomatic patients and the time required for the study, have limited its clinical application.
Although plaque characteristics and evidence of silent emboli may help predict patients at highest risk for subsequent events related to carotid bifurcation stenosis, their absence does not infer minimal stroke risk in patients with otherwise clinically significant carotid bifurcation disease. There are currently no data to support these endpoints as superior to the more straightforward measurements of percent stenosis and plaque progression for selecting patients for carotid intervention. The major clinical trials did not consistently collect data on either plaque characteristics or evidence of silent emboli; therefore, it is impossible to determine what effect these may have had on their outcomes. The ability to reliably characterize a vulnerable plaque using techniques that are readily reproducible and widely available has not been established and is an area of active investigation. Similarly, although detection of previous embolic plaque activity in a neurologically asymptomatic patient using brain imaging or TCD monitoring has been associated with increased stroke risk, absence of these features has not been shown to imply minimal stroke risk in patients who have a high grade stenosis. It is hoped that use of these modalities in future clinical trials will put their clinical value in perspective. At present, however, the most reliable imaging predictor of stroke risk remains the accurate measurement of diameter stenosis. Factors associated with increased stroke risk are listed in Table 99-2.
Table 99-2
Clinical Factors Associated with increased Stroke Risk
| Plaque Characteristics | Patient Characteristics |
| Diameter stenosis* | Neurologic symptoms |
| Plaque ulceration | H/O contralateral stroke* |
| Plaque progression | Contralateral carotid occlusion* |
| Echolucent plaque* | Renal insufficiency* |
| Plaque area* | Smoking* |
| Disrupted fibrous cap | Clinical silently emboli by TCD or MRI/CT |
| “Discrete white areas” within plaque* | |
| Active inflammation by MRI |
Impact of Patient Specific Features on Clinical Decision Making
There are a number of factors to consider before recommending intervention for a specific carotid lesion. Some of these are generic and relate to the decision whether or not to recommend any intervention in addition to BMT, whereas others are specific to the carotid intervention being considered (CAS or CEA).
Factors Influencing the Decision to Recommend Intervention
Life Expectancy
The patient’s longevity is a prime consideration in deciding on the role of intervention, particularly in asymptomatic patients. In NASCET, ECST, and ACAS, only patients who had a life expectancy of 5 years were eligible for enrollment. In the symptomatic studies,1–3 patients in the medical arm with more than 70% stenosis had a stroke risk of 24.5% at 2 years, whereas patients with lesser degrees of stenosis (50%-69%) had a 2-year stroke risk of 14.6%. In the asymptomatic studies, the risk of stroke in the medical arms was considerably less (11% in ACAS and 11.8% ACST at 5 years).4,5 It follows from these observations that symptomatic patients with high grade stenosis will benefit almost immediately from intervention to reduce stroke risk, whereas symptomatic patients with lesser degrees of stenosis have a lesser benefit, and asymptomatic patients will need to live a considerable period of time to see the benefit of a cumulative reduction in stroke risk. In general, asymptomatic patients should be expected to survive at least 3 to 5 years to derive significant benefit in stroke reduction from CEA.
Age
In addition to the impact that age has on life expectancy, there is a complex interaction of age with the decision to recommend intervention. NASCET, ECST, and ACAS excluded patients older than 80 years, whereas ACST permitted randomization up to age 91 years. However, the number of patients older than 80 years in ACST was low, and reliable subgroup analysis of the effect of advanced age on outcomes was not possible.5 There are data demonstrating that age itself is not associated with increased risk of adverse events after CEA.34–36 In contrast, age does seem to influence outcomes after carotid angioplasty and CAS. In the lead-in phase of the CREST study, there were early indications that CAS was associated with increased risk of periprocedural stroke,37 and ultimately, CREST demonstrated that increasing age was associated with an increase in neurologic events in the CAS arm but not in the CEA arm.34 Although the effect of age on the combined endpoints in CREST was reached at approximately age 70 years, the age at which stroke rates (as opposed to the composite endpoint) increased with CAS was 64 years.34 Multiple other reports indicated that older patients are at significantly higher risk of stroke, and to a lesser degree death, after CAS. Khatri et al38 analyzed the Nationwide Inpatient Sample from 2005 to 2008 and found an increased risk of stroke in patients older than 70 years who underwent CAS (odds ratio [OR], 1.7%), whereas Jim et al35 used the Society for Vascular Survey (SVS) Registry and found that CEA was superior to CAS in patients older than 65 years. A meta-analysis of the SPACE, ICSS, and EVA3-S trials, which compared CAS with CEA in symptomatic patients, revealed that the majority of increased risk of stroke after CAS was in the patient group ages 70 years or older.39 The explanation for this observation is likely multifactorial. Tortuosity of the aortic arch and great vessels is more common in older patients, and such tortuosity has been associated with an increased risk of stroke after CAS.40 In addition, increasing age has been shown to be associated with increasing plaque instability.41 Advancing age may simply increase the risk for anatomic conditions associated with worse outcomes after CAS.
Gender
Most of the major randomized trials have found a greater benefit from CEA in men compared with women.1–5 This difference has been ascribed both to an overall increased risk of stroke in female patients after CEA and a reduced long-term stroke risk in women compared with men in the medical arms of these trials. Women are generally recognized to have smaller carotid arteries than men and more often require patching after CEA. There were no standard criterion for patching after CEA in any of the major carotid trials, and routine patching after CEA was not standard of care during the time those trials were performed. It is impossible, therefore, to know the effect inconsistent patching may have had on the postoperative results in the female cohort of these studies. NASCET2,6 and ECST3 both reported this gender-based difference, although CEA was still beneficial in women with 70% to 99% stenosis. ACAS did not find a benefit for CEA in women,4 although the number of women enrolled in the trial was small. The larger ACST trial,5 with almost double the enrollment of ACAS, found benefit for CEA in both men and women. Some recent population-based studies comparing CAS and CEA in large databases, suggested that CAS might be associated with more complications than CEA in women. Rockman et al42 studied the National Inpatient Sample, which included 54,658 procedures, and found that stroke was more common in women after CAS than CEA in both the symptomatic (3.4% versus 6.2%; P = .01) and asymptomatic (0.9% versus 2.1%; P = .001) cohorts. Vouyouka et al43 reviewed data in 20,613 carotid interventions in women between 2007 and 2009 from New York and Florida, and found that stroke and death rates were lower for CEA than CAS in both asymptomatic (1.7% versus 3.1%) and symptomatic (3.8% versus 10.9%) women. In a propensity-matched analysis of CAS and CEA, the benefit of CEA remained significant for symptomatic but not asymptomatic women.43
Functional Status
Because carotid intervention is intended to prevent stroke, overall patient functional status is an important element in decision making, both from the standpoint of deriving benefit from a prophylactic treatment and from the perspective of elevated procedural risk inherent with poor functional status. The patient should have a baseline level of function such that a further neurologic event would result in serious deterioration in functional or cognitive activity. Patients with dense hemispheric neurologic deficits are unlikely to benefit from further intervention. Similarly, patients who have significant dementia and limited functional activity should only undergo intervention after very detailed and careful discussions with the patient, family, and another physician familiar with the patient. There is no doubt that intervention in patients with mild to moderate disability can be justified in an attempt to maintain function, but these decisions are often complex, and an unbiased assessment of the patient’s functional status and future course is imperative to avoid unnecessary intervention. Poor functional status, as reflected particularly in cardiac, renal, and pulmonary impairment, has been correlated with increased perioperative risk. In addition, a recent report noted that, even when perioperative complication rates are low, patients with impaired functional status who successfully undergo intervention are at a higher risk of adverse events during follow-up, limiting the effectiveness of operation for asymptomatic stenosis in this patient group.44
Cardiac Status
The major non-neurologic complication after CEA is myocardial infarction. Although the rate of clinical myocardial infarction has been low in the major trials, recent studies have shown that CEA is associated with an increased risk of overall cardiac events, including subclinical infarction, compared with carotid stenting.45–47 It is this increased rate of myocardial events that provide the equivalent rates of overall cardiovascular events seen in the CREST trial.45 There is evidence that perioperative cardiac events are associated with late mortality.48 Therefore, it is important to evaluate the cardiac status of patients before recommending intervention. Patients with evidence of uncompensated congestive heart failure or untreated significant coronary disease will be more likely to experience cardiac events.49 The extent of cardiac evaluation in each clinical scenario varies with the imputed benefit of intervention over medical therapy. In symptomatic patients with severe stenosis, only a history and physical examination may be required to detect unstable cardiac conditions, whereas in asymptomatic patients, a full cardiac workup, including evaluation for subclinical coronary ischemia, should be strongly considered. The benefit of intervention over medical therapy in asymptomatic patients is small enough that any potential cardiac source of morbidity or mortality should be identified and assessed before a decision to proceed with intervention is undertaken. Attention to these details will optimize the results of intervention, whereas failure to consider them sufficiently may result in suboptimal results by intervening in “high risk” asymptomatic individuals as was done in the SAPPHIRE trial.46
Pulmonary Disease
In general, pulmonary disease does not play a major role in morbidity after carotid intervention. Both CEA and CAS may be performed under local and/or regional anesthesia, and even when CEA is performed under general anesthesia, pulmonary complications are infrequent. However, any active pulmonary disease should be stabilized before intervention is undertaken, and the effect of pulmonary disease on longevity should be considered.
Renal Insufficiency
Moderate to severe renal insufficiency reduces life expectancy and is associated with increased complication rates after both CEA and CAS.50,51 Chronic kidney disease has also been associated with features of increased plaque instability on histologic examination and an increased incidence of cerebrovascular events before surgery.52 Renal insufficiency was an independent risk factor for subsequent stroke in the ASCRS study.7 Chronic renal insufficiency has been associated with increased complication rates after both CEA53,54 and CAS.55,56 As a consequence, no clear recommendations on the impact of renal insufficiency on decision making for the type of intervention can be made.
Contralateral Carotid Occlusion
NASCET identified increased perioperative stroke risk in patients with CCO as 5.8% to 14%.6 However, a number of single-center studies have not confirmed this increased risk,57–60 and a meta-analysis of the literature suggests a statistically significant but small (2.4% compared to 3.7%) increase in perioperative stroke rates in CCOs after CEA.61 CCO may identify a patient group with a history of clinical or subclinical previous contralateral ischemia or may simply represent patients with an overall higher burden of disease. It is also possible that good results in reports from single-center series reflect more standardized patient selection and a standardized approach to details of the operation, such as the use of a shunt and patch, all of which may influence outcome. Recently, results of both CEA and CAS in patients with CCOs were compared in patients entered into the SVS Carotid Registry database.62 This comparison demonstrated a small but statistically significant increased risk of stroke after CEA (3.15% versus 1.06%) in the presence of carotid occlusion, supporting the findings of the meta-analysis. The stroke risk in the CAS group was not affected by the presence of contralateral occlusion (2.1% versus 2.3%). It is relevant that in the data from the SVS Carotid Registry, which included both symptomatic and asymptomatic patients, the overall stroke rate in CEA patients with a contralateral occlusion still fell within the American Heart Association (AHA) guidelines and was not statistically different from the stroke rate in CCO patients who underwent CAS (CEA 3.15%; CAS 2.13%; P = .2).62 Thus, it appears that contralateral occlusion does slightly increase stroke risk after CEA but not after CAS; however, the increased stroke risk after CEA is modest. Results in patients with CCOs appear to be similar after both CEA and CAS. As noted earlier, data on stroke risk in patients with contralateral occlusion treated with BMT are conflicting.
Factors That Influence The Choice of Specific Intervention
Presence of Neurologic Symptoms
The preponderance of data has shown that results with CAS are inferior to CEA in neurologically symptomatic patients. This has been true in the randomized trials of symptomatic patients comparing CAS and CEA63–65 and in the subanalysis of symptomatic patients in the CREST trial.66 This position is reflected in all of the societal guidelines published to date (see the following). Of note, a recent publication combining the results of the three large European trials comparing CEA and CAS in symptomatic patients suggested that the benefit of CEA over CAS (stroke rate 2.8% versus 9.4%; hazard ratio [HR], 3.4) is particularly high in the first week after symptom onset.67 This is particularly relevant because most guidelines currently recommend early carotid intervention after stroke (see the following).
Hostile Neck
This is defined by neck anatomy with poor tissue planes or increased risk of postoperative infection. Such circumstances occur most frequently after major neck surgery involving dissection within the carotid sheath, radiation to the neck, or presence of a stoma in the cervical region. Each of these circumstances can increase the complication rate after CEA. It is important to note that a history of radiation, stoma, or surgery alone does not, by itself, define a “hostile neck”; rather, it is the degree of local change in the tissues that surround the carotid artery that is relevant. There are series reporting excellent results with CEA for recurrent carotid stenosis68 and after cervical radiation.69 These series likely include patients who are appropriately selected and operated upon in centers of excellence. It is generally accepted that cranial nerve injury is increased when CEA is performed for restenotic lesions, and this is likely also true with radiated fields. Increased wound complications after radiation and/or in the presence of a tracheotomy are well known. In general, these conditions represent a relative increased risk for CEA.
Lesions Outside the Area of the Cervical Carotid Artery
Lesions that are below the clavicle or above the level of the second cervical vertebra also present challenges for open surgical repair. These lesions are infrequently encountered in practice. Tandem lesions involving the proximal common carotid artery may be treated with CEA combined with retrograde stenting with good results.70 However, high lesions, requiring mandibular subluxation or other maneuvers to increase surgical exposure (see Chapter 100) may result in an increased incidence of cranial nerve injury after CEA.71 Intervention in these lesions should be carefully considered, particularly in the asymptomatic patient.
Vessel Tortuosity
Tortuosity of any of the vessels that must be traversed for placement of a carotid stent may represent increased risks for CAS. This is rarely an issue with the ileofemoral system; however, tortuosity of the aortic arch, aortic arch atheroma, and tortuosity of the proximal carotid vessels all increase the difficulty of establishing a stable platform for CAS and increase the risk of embolization with CAS.40,72 Some surgeons have tried to address this by transcervical CAS.73 The role of transcervical stenting is an area under clinical investigation.74 Its relative benefit, compared with CEA, remains unproven. Tortuosity of the distal internal carotid artery may also present challenges for CAS.40 Such tortuosity may complicate stable accurate placement of a distal embolic protection device. Most authorities believe embolic protection strategies are an important component of CAS, and several studies have shown improved results with CAS when such devices are used.75
Lesion Character
Lesion character has been briefly discussed previously and is addressed in detail in Chapter 97. There are data that suggest that lipid rich, echolucent lesions (i.e., vulnerable plaque) are more likely to result in emboli after CAS than after CEA. These observations are supported by the results of randomized studies comparing CEA and CAS in symptomatic patients (who by definition are more likely to have vulnerable plaque) who repeatedly show higher risks of stroke after CAS. In addition, lesions that are more than 15 mm in length, preocclusive highly calcified, or contain fresh thrombus have all been associated with worse outcomes after CAS.40,75
Treatment Options in Patients with Carotid Bifurcation Stenosis
With the preceding information, a therapeutic approach can be chosen for a particular patient based on the risk of their clinical condition, their longevity, and the relative risks of intervention. There are considerable data addressing the role of intervention, specifically CEA and BMT compared with BMT alone in stroke prevention. There are also a number of studies addressing the relative benefits of CEA and CAS in patients who were deemed appropriate for intervention. However, there are no data directly comparing CAS and BMT and limited contemporary data comparing “contemporary” BMT and “contemporary” CEA.
Risk Factor Reduction and Medical Management
A variety of clinical conditions have been associated with increased stroke risk: hypertension, diabetes, hyperlipidemia, smoking, and alcoholism. Efforts to control these conditions have been shown to reduce stroke risk in large population studies. In addition to pharmacologic interventions, treatment with antiplatelet agents and use of statins has been shown to reduce long-term stroke risk. These efforts form the cornerstone of the medical management of all patients at risk for stroke. Many of these interventions not only reduce stroke risk, but overall cardiovascular morbidity and mortality. The current data on each of these are briefly summarized and have been recently re-capitulated in a set of guidelines by the AHA.76 BMT should be provided to all patients with carotid stenosis, stroke, or other manifestations of cardiovascular risk. The cornerstones of BMT are listed in Table 99-3.
Table 99-3
Effect of Risk Reduction and Best Medical Therapy on Stroke and Cardiovascular Events
| Treatment | Goal | Comments |
| Antiplatelet therapy | Either single or dual drug acceptable | Reduces both stroke rate and overall MACEs |
| Antihypertensive therapy | Decrease BP by 10 mm Hg systolic/5 mm Hg diastolic or to 120/80 mm Hg in hypertensive patients | Reduces stroke recurrence Treat all patients regardless of baseline BP, after first 24 hr |
| Diabetes mellitus | Aim for HgbA1c <7 | Reduce overall stroke rate, no benefit in tight control |
| Smoking cessation | Total abstinence | Reduces stroke and MACEs |
| Statin therapy | Reduce LDL by 50% or <70 mg/dL | Treat hyperlipidemia and normolipemic patients with H/O stroke, may be beneficial before CEA/CAS |
| Alcohol | Avoid excessive consumption |
BP, Blood pressure; CAS, carotid artery stenting; CEA, carotid endarterectomy; HgbA1c, glycosylated hemoglobin; H/O, history of; LDL, low-density lipoprotein; MACEs, major adverse cardiovascular events.
Blood Pressure Control
Control of blood pressure has been shown to reduce the overall risk of stroke and risk of recurrent stroke in a number of studies.77,78 This beneficial effect is seen in both hypertensive patients and those whose baseline blood pressure falls within the accepted guidelines. The one exception to this occurs in the first 24 hours after an acute stroke, where aggressive reduction of blood pressure should be avoided to optimize cerebral perfusion pressure. Efforts to reduce blood pressure after the first 24 hours should be made in all patients after stroke, with absolute targets of 10 mm Hg systolic and 5 mm Hg diastolic in normotensive patients or a blood pressure of 120/80 mm Hg in hypertensive patients.76 There is no definitive benefit of one class of antihypertensive agents over another for stroke reduction.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree