Chapter 100
Carotid Artery
Endarterectomy
Maggie Arnold, Bruce A. Perler
Based on a chapter in the seventh edition by Glen S. Roseborough and Bruce A. Perler
The pathogenesis of ischemic stroke related to carotid bifurcation atherosclerosis was described in 1951 by Dr. C. Miller Fisher, a noted Boston neurologist. In his sentinel report he speculated that surgical removal of the offending plaque, if feasible, could prevent stroke. Shortly thereafter, the era of carotid surgery began in 1954 when Eastcott et al1 published a case report documenting the first successful reconstruction of the carotid artery to treat symptomatic carotid occlusive disease in a woman with recurrent transient ischemic attacks (TIAs). Her treatment consisted of excision of the carotid bifurcation, ligation of the external carotid artery (ECA), and reconstruction with direct anastomosis of the common carotid artery (CCA) to the internal carotid artery (ICA). DeBakey et al2 had performed a successful carotid endarterectomy (CEA) the year before but did not publish their experience until 1959 in a paper that encompassed surgical management of occlusive disease of the carotid, vertebral, and aortic arch vessels. In this prescient paper they documented numerous technical approaches to managing carotid occlusive disease, including eversion endarterectomy, patch angioplasty, and even shunting.
Performance of CEA experienced remarkable growth in the 1970s and 1980s after several natural history studies demonstrated that carotid stenosis was an ominous risk factor for disabling stroke and death3–6 and after a randomized multicenter trial published in 1969 showed that carotid surgery reduced the incidence of stroke from symptomatic carotid lesions.7 However, other studies showed that complication rates were often high, thus compromising the potential benefit of CEA.8 Randomized controlled studies performed in the 1990s definitively established the safety and efficacy of CEA and its superiority over the best medical management of patients with symptomatic and asymptomatic carotid disease.9–12
Epidemiology
Incidence
Cerebrovascular disease is the second leading cause of death worldwide and is responsible for approximately 9.5% of all deaths. At least 750,000 strokes occur annually in the United States. Approximately 15% of strokes are fatal, 15% to 20% are severely disabling, and another 15% to 20% of patients who have stroke and recover have a subsequent disabling stroke in the future. Approximately 80% of these strokes are ischemic, and atherosclerotic disease of the cervical carotid artery is responsible for at least 40% of ischemic strokes13 (see also Chapter 97).
Etiology
Strokes secondary to carotid artery stenosis are a consequence of atheroembolization, or thromboembolism in the case of an occluding artery and distal propagation of thrombus. Small pieces of the carotid bifurcation plaque break off and embolize to the intracranial vessels, usually to the middle cerebral artery (MCA) in the anterior circulation. These strokes can also result from lesions in the common carotid artery (CCA) or in the distal or intracranial portion of the internal carotid artery (ICA). Strokes can also be secondary to a low flow state through the carotid artery (see Chapter 97).
Indications for Carotid Endarterectomy
The indications for carotid endarterectomy (CEA) are presented in Chapter 99. Appropriate indications for intervention have been solidified by level I evidence derived from several randomized prospective clinical trials.14 On the basis of the latest evidence-based guidelines published by the Society for Vascular Surgery, CEA is recommended for most symptomatic patients with 50% to 99% ICA stenosis. Further, CEA should be considered for patients with 60% to 99% asymptomatic ICA stenosis if the patient’s perioperative stroke and death rate is less than 3% and if the patient has at least a 3- to 5-year life expectancy. In the symptomatic patient with a 50% to 99% ICA stenosis and either medical or anatomic contraindications to CEA, carotid artery stenting (CAS) should be considered. Medical therapy, rather than CEA or CAS, should be selected in symptomatic patients with a stenosis less than 50% and for asymptomatic patients with stenoses less than 60%.14 Likewise, medical therapy is advised for asymptomatic patents with greater than 60% stenosis and high risk factors for CEA.14
Preoperative Imaging
Documentation of carotid disease with appropriate imaging is critical before CEA is performed. Options for imaging the carotid artery include duplex ultrasound (DUS), digital subtraction angiography (DSA), computed tomographic angiography (CTA), and magnetic resonance angiography (MRA). Formerly the “gold standard,” catheter-based angiography is seldom required in contemporary practice in a purely diagnostic mode. The most appropriate use of these imaging modalities is thoroughly reviewed in Chapter 98. There are varying opinions with respect to the most accurate noninvasive imaging modality for evaluation of carotid artery disease, with some series suggesting that DUS and others indicating that either MRA or CTA is the most accurate.15–21 Still others have observed that MRA tends to overestimate and CTA underestimate the degree of stenosis. In fact, the Clinical Practice Guidelines published in 2011 by the Society for Vascular Surgery (SVS) recommend that DUS in an accredited laboratory is the most appropriate first test to evaluate the patient with suspected carotid artery disease, with either CTA or MRA reserved for patients with suspected disease proximal or distal to the neck, or when there are indeterminate DUS findings or other confounding issues. Further, the guidelines indicate that when there is definitive evidence of 50% to 99% stenosis in a symptomatic patient or 70% to 99% stenosis in an asymptomatic patient, the DUS findings are sufficient to proceed to intervention.14 Many surgeons proceed to endarterectomy on the basis of DUS findings alone, although it is not possible to determine how often this practice occurs. 22 Nevertheless, it does appear that in contemporary practice, DUS is the most popular preoperative imaging modality and vey likely the sole test performed prior to CEA. For example, in a report from the Vascular Study Group of Northern New England of 6143 CEAs, DUS was performed in 96% of the cases, and MRA, CTA, and arteriography in only 25%, 17%, and 9%, respectively. It is clear that the majority of patients likely underwent CEA on the basis of a DUS scan alone.23 Each practitioner should assess the relative accuracy of these imaging modalities in his or her own institution. Clearly, if the patient is found to have an intermediate stenosis and is asymptomatic, one should perform at least another noninvasive test to confirm this finding before recommending CEA. If there is discordance between findings of these studies, one should either obtain a third noninvasive test or proceed to angiography.
Perioperative Medical Management
The medical management of carotid disease has evolved and improved significantly over the last decade. Risk stratification is important in determining the most appropriate preoperative cardiac workup and perioperative management of the patient with vascular disease, including the patient undergoing CEA. The American Heart Association’s (AHA) perioperative guidelines classify CEA as an intermediate-risk procedure.24 Preoperative cardiac assessment for patients undergoing intermediate-risk procedures generally consists of a 12-lead electrocardiogram (ECG), with further testing (i.e., stress test) if the patient has three or more clinical risk factors (such as ischemic heart disease, history of compensated heart failure or prior history of heart failure, diabetes mellitus, and renal insufficiency) or a poor functional capacity. The perioperative medical management of the patient undergoing CEA should include blood pressure control as well as beta-blocker, statin, and antiplatelet therapy.
Beta Blockers
On the basis of early small clinical trials, it was believed that all patients with vascular disease would benefit from perioperative beta blockade. Several large trials have been published, however, that challenge this recommendation. The largest of these, the Perioperative Ischemic Evaluation Study (POISE trial), randomly allocated patients undergoing noncardiac surgery to receive metoprolol or placebo preoperatively as well as for 30 days postoperatively. The investigators found a significant reduction in the number of myocardial infarctions (MIs) in the metoprolol group (4.2% vs. 5.7%). However, the group also found that the patients in the metoprolol group had increased overall mortality and stroke rate (3.1% vs. 2.3% and 1.0% vs. 0.5%, respectively).25 On the basis of the findings of this pivotal trial and other data, the AHA issued a revised statement on the issue of perioperatively beta blockade. The current recommendation is to continue beta blockers in patients who are already taking them. There is a weak recommendation to start beta blockers if a patient has a history of coronary artery disease (CAD) or more than one clinical risk factor. For patients who are undergoing vascular surgery (including carotid endarterectomy) who have a single risk factor in the absence of documented CAD, there is a very weak recommendation to start beta blockade.26 Perioperative beta blockade, with a goal of achieving a maximum heart rate of 60 to 80 beats per minute, is recommended in the SVS carotid guidelines.14
Antiplatelet Therapy
One of the most significant improvements in the management of cardiovascular disease in general has been the aggressive use of antiplatelet therapy, and early studies indicated a benefit of antiplatelet therapy in the perioperative period among patients undergoing CEA. Meta-analyses of antiplatelet therapy trials published by the U.K. Antiplatelet Trialists’ Collaboration in 1994 and 2002 concluded that antiplatelet therapy significantly reduces the incidence of stroke in high-risk patients, with a resultant 25% reduction in strokes overall.27,28 The majority of these studies involved the use of aspirin or aspirin plus another antiplatelet agent. The benefit of acetylsalicylic acid (ASA) in reducing postoperative stroke and death after CEA was first shown in a secondary analysis from the North American Symptomatic Carotid Endarterectomy Trial (NASCET), a randomized controlled trial (RCT) that demonstrated the benefit of CEA in symptomatic patients with at least 50% ICA stenosis.9 In that trial, patients were prescribed up to 1300 mg ASA daily if tolerated. A subsequent association was found between perioperative stroke and death and the amount of ASA taken before surgery. The risk for perioperative stroke and death was 1.8% in patients taking 650 to 1300 mg ASA daily versus 6.9% in patients taking 0 to 325 mg daily. An RCT was subsequently conducted in which 232 patients undergoing CEA were randomly assigned to receive either ASA 75 mg or placebo preoperatively and for 6 months postoperatively.29 Stroke at 30 days and at 6 months occurred in 0 and 2 ASA-treated patients, versus 7 and 11 placebo patients, respectively (P < .01), with no difference in bleeding complications.
To determine the optimal daily dose of preoperative ASA in reducing stroke and death associated with CEA, the ASA and Carotid Endarterectomy (ACE) trial randomly allocated 2849 patients to ASA doses of 81, 325, 650, or 1300 mg started preoperatively and continued for 3 months.30 The combined end point of 30-day stroke, death, or myocardial infarction after CEA occurred in 5.4% of patients in the low-dose groups (81 and 325 mg) versus 7.0% of patients in the high-dose groups (P = .03). In an efficacy analysis focusing on patients not previously taking high-dose ASA, the combined end point was seen in only 3.7% of patients in the low-dose groups versus 8.2% of patients in the high-dose groups at 30 days (P = .002). Fewer hemorrhagic complications were also seen in the low-dose ASA groups, but this difference was not significant. One should note that a placebo group was not deemed appropriate for this study because of the widely accepted benefit of ASA in preventing stroke.
Other antiplatelet agents have not been as extensively studied in terms of their benefit in stroke reduction after CEA, but later studies of clopidogrel provide evidence that perioperative embolization is decreased. In one trial, 100 patients undergoing CEA who were taking 150 mg ASA daily were randomly assigned to receive in addition either clopidogrel 75 mg or placebo the night before surgery.31 The number of emboli detected by transcranial Doppler ultrasonography (TCD) within 3 hours of CEA was the main outcome measure. There was a tenfold reduction in the number of patients who experienced more than 20 microemboli in that period (odds ratio [OR] 10.2; 95% confidence interval [CI] 1.3 to 83.3; P < .01) in the group taking clopidogrel combined with ASA. No increase in bleeding complications or transfusion requirements was noted. The embolization rate was used as a surrogate for stroke because stroke occurs infrequently and the embolization rate has been well correlated with stroke risk during CEA. Other antiplatelet agents, such as ticlopidine and glycoprotein IIb/IIIa antagonists, though demonstrated to reduce stroke risk in nonsurgical patients, have not been specifically studied during CEA and generally have higher risk profiles than ASA and clopidogrel. On the basis of this level I evidence, and in light of the overall cardiovascular benefits, it is possible to recommend ASA, clopidogrel, or both for use before and after CEA.
On the other hand, a 2004 meta-analysis published several years ago found that antiplatelet therapy was not effective in preventing stroke and other vascular events after CEA.32 Further, a survey of the members of the Vascular Society of Great Britain and Ireland as well as the European Society for Vascular Surgery found for symptomatic patients, 5% (20/392) would stop aspirin and 43% (170/392) would stop clopidogrel before surgery. For asymptomatic patients, 12% (47/392) would stop aspirin and 55% (217/392) would stop clopidogrel.33 Nevertheless, the preponderance of evidence strongly supports the efficacy and safety of antiplatelet therapy in the perioperative period for patients undergoing CEA. For example, in a multistate quality improvement study of Medicare patients undergoing 10,561 CEA procedures, failure to administer antiplatelet therapy was identified as a risk factor for perioperative stroke.34 Likewise, in a report of 2714 patients undergoing 3092 CEA procedures in 11 hospitals in northern New England, the administration of preoperative antiplatelet therapy was associated with a 60% reduction in the risk of perioperative stroke.35 These findings were corroborated in another study that demonstrated an absolute 7% reduction in the incidence of stroke in the early postoperative period.36 In fact, on the basis of a review of the totality of evidence, the 2011 Clinical Practice Guidelines of the SVS recommend that all patients undergoing CEA should receive aspirin (81-325 mg) perioperatively.14
Heparin
Heparin has been administered as therapy for acute stroke or crescendo TIAs to patients before they undergo CEA. The International Stroke Trial did not find any benefit of routine heparin administration for acute stroke because of increased numbers of hemorrhagic stroke and fatal extracranial bleeding.37 However, a report from the Oregon Health Sciences University found that crescendo TIAs could be controlled with intravenous heparin, thereby allowing urgent CEA to be delayed.38
Unfractionated heparin (UFH) is routinely used intraoperatively to prevent carotid thrombosis despite a lack of level I evidence to support this practice. The combination of aspirin and intraoperative heparin administration appears to be especially effective in preventing thrombosis.39 The University of Rochester group found that low-dose intraoperative heparin is safe and can obviate the use of protamine to reverse heparin intraoperatively.40
Several studies have looked at the effects of UFH on platelet aggregation. Investigators found that platelet aggregation increased tenfold in response to arachidonic acid after administration of UFH even though the patients were taking aspirin.41 With this finding in mind, the investigators then studied low-molecular-weight heparin (LMWH) as an alternative to UFH. A randomized controlled trial looked at the rate of perioperative embolization via TCD for patients given either UFH or low-molecular-weight heparin. They found that the odds ratio for experiencing a higher number of emboli in the first 3 hours after CEA was twice that of patients receiving UFH (P = .04). However, despite the fact that the UFH group experienced more emboli, they did not have a higher 30-day stroke or death rate.42 It should be noted that this was a small study and that the clinical end points for the two groups were not different.
Protamine Administration
Numerous publications have examined whether reversing heparin with protamine during CEA is safe. The only randomized trial that looked at this question was a small study consisting of 64 patients. It demonstrated significantly reduced wound drainage with protamine but a trend toward an increased rate of stroke and death from carotid thrombosis.43 Treiman et al44 retrospectively compared their experience with 328 patients who received protamine and 369 patients who did not over a 5-year period and found no difference in stroke rates but a significantly higher incidence of wound hematomas in patients who did not receive protamine. Similar results were reported in a retrospective study of 407 CEAs by Levison et al.45 However, in another retrospective study involving 348 patients, Mauney et al46 found a significantly higher stroke rate with protamine (2.6% vs. 0%, P < .045) and no significant increase in bleeding rate in patients who did not receive protamine. In a publication of observations of protamine use during the General Anesthesia versus Local Anesthesia for Carotid Surgery (GALA) trial, protamine was not found to be associated with stroke.47 Likewise, the Vascular Study Group of New England, reporting on 4587 CEA procedures performed by 66 surgeons in 11 hospitals from 2003 through 2008, found that protamine administration was associated with a reduced incidence of serious bleeding requiring reoperation without increasing the risk of perioperative stroke, myocardial infarction, or death.48 The preponderance of evidence now suggests that protamine administration does not increase perioperative stroke risk, although we prefer not to use it routinely unless there is truly unusual bleeding.
Dextran
Dextran is a polysaccharide that inhibits platelet aggregation.49 It has been used to control embolic episodes both preoperatively and postoperatively. British investigators in 1997 showed that a 6-hour dextran infusion effectively controlled postoperative embolic events as measured by TCD, with a 0% stroke and mortality rate in a series of 100 patients.50 In a follow-up study, the same group found that a 3-hour infusion was just as effective as a 6-hour infusion.51 In a study of 19 patients with recurrent or crescendo TIAs and high-grade carotid stenosis, dextran was used effectively preoperatively to control both clinical symptoms and embolic events, as measured by TCD, before CEA.52 The same investigators showed dextran infusion and TCD to be cost-effective in preventing stroke.53 We favor dextran infusion after CEA to control platelet aggregation on the endarterectomy site and potential microembolization. However, dextran should be used cautiously, especially among patients with cardiac disease. Farber et al,54 for example, found that postoperative dextran infusion was associated with a significantly increased incidence of perioperative MI and congestive heart failure but not a reduced rate of perioperative stroke.
Statins
The potential benefits of statins in patients with carotid artery disease are several. A reduction in cholesterol levels with statins may be associated with plaque regression (see Chapter 29).55–63 For example, the Asymptomatic Carotid Artery Progression Study (ASAPS) demonstrated that lovastatin was associated with reduced carotid artery intima-media thickness and also a lower rate of combined cardiovascular events, although not a lower stroke rate.64,65
However, numerous trials conducted since the mid-1990s have demonstrated statin medications to be highly effective in primary and secondary stroke prevention.65–70 Furthermore, it appears that the stroke prevention benefits of statins are related to their pleiotropic effects rather than their cholesterol-lowering effects. In addition, several studies have demonstrated that statins are associated with reductions in the rate of perioperative cardiac morbidity and overall mortality in patients undergoing major vascular surgical procedures.71–74
The demonstrated benefit of statins in primary and secondary stoke prevention and their positive impact on the incidence of perioperative cardiac complications and mortality in patients undergoing major vascular surgery stimulated our group to investigate the potential influence of statin medications specifically on the outcome of CEA. In a series of 1566 patients undergoing CEA, we found that statins were associated with reduced 30-day incidences of stroke (1.2% vs. 4.5%, P = .002), TIA (1.5% vs. 3.6%, P = .01), and death (0.3% vs. 2.1%, P = .002). Multivariable analysis demonstrated statins to be associated with a threefold lower risk of stroke (P = .019) and fivefold lower risk of death (P = .049) 30 days after surgery.75 In a large administrative database analysis from Canada, Kennedy et al76 reported a significantly lower rate of perioperative stroke and death in symptomatic patients who were taking statin medications at the time of CEA. Furthermore, Lamuraglia et al77 reported that lipid-lowering agents were associated with significant protection against recurrent carotid stenosis or late anatomic failure after CEA. The evidence seems clear that all patients undergoing CEA should be taking statin medications at the time of surgery and long-term.
Operative Technique
Anesthesia
A fundamental consideration in the conduct of CEA is selection of the anesthetic method. CEA may be performed with use of general anesthesia (GA), regional anesthesia (RA) with deep or superficial cervical block, and even pure local anesthesia (LA). Although early reports suggested a reduced length of hospital stay associated with CEA performed with RA, comparable lengths of stay are routinely documented today in patients who undergo surgery under GA. The majority of studies comparing the two techniques have reported improved perioperative cardiac stability with RA, but this improvement does not necessarily result in a reduced incidence of MI.78,79
Disadvantages of RA include patient discomfort or anxiety, risk of seizure or allergic reaction, anxiety for the operating surgeon, and compromise of technique in a teaching setting. A meta-analysis by Tangkanakul et al,80 as well as one by Rerkasem et al,79 showed no clear benefit for CEA performed with use of LA. The GALA trial was a prospective European multinational study carried out in 95 hospitals in 24 countries, and involved 3526 patients who underwent CEA under general (1753) or local (1773) anesthesia from June 1999 through October 2007. There was no significant difference in the incidence of the primary outcome measure of stroke, death, or MI at 30 days, which occurred in 84 (4.8%) patients who underwent CEA under GA and 80 (4.5%) patients who underwent CEA under LA.81 There was also no difference in hospital length of stay between the anesthesia groups. There was a trend toward lower stroke rate among patients with a contralateral carotid occlusion having surgery under local anesthesia, although this difference was not statistically significant.
The main benefit of LA is that it facilitates efficient selective shunting if that is the surgeon’s preference. However, the benefit of selective shunting is unclear (see under “Shunting”). In addition, there may be subsets of patients with significant cardiac or pulmonary comorbidity for whom having CEA under local or regional anesthesia is associated with less risk. For example, in an analysis of CEA performed by 64 surgeons in six hospitals in New York State, there was a 70% risk-adjusted risk of perioperative stroke or death among patients undergoing CEA under LA.82 In another analysis of the National Surgical Quality Improvement Program (NSQIP) database, which included 13622 CEA procedures performed at 123 Veterans Administration and 14 private hospitals, RA was utilized in 2437 (18%) cases and was associated with relative risk reductions for stroke (17%), death (24%), and cardiac complications (33%).83
Patient Positioning
Careful positioning of the patient is important to ensure patient comfort and adequate operative exposure. Positioning begins with placing a roll behind the scapulae to achieve some hyperextension of the neck. A padded ring is placed under the head to prevent neck injury from extreme hyperextension. If GA is used, the endotracheal tube should be taped to the corner of the mouth opposite the surgical field. If LA or RA is used, a Mayo stand is placed over the patient’s head to suspend the surgical drapes away from the patient’s face to prevent a sensation of claustrophobia. It is our practice to tape the fan for a Bair Hugger warmer (Arizant Healthcare Inc., Eden Prairie, Minnesota) on the underside of the Mayo stand, because blowing air on the patient’s face relieves discomfort. If GA is used, it is induced before placement of additional lines. A radial artery catheter is inserted for continuous blood pressure monitoring.
Skin Incision
One of two skin incisions may be used (Fig. 100-1). The standard incision is a longitudinal incision parallel to the medial border of the sternocleidomastoid muscle. The upper portion of the incision is angled posterior to the earlobe if cephalic exposure above the angle of the jaw is required. An alternative method is to place the incision in an appropriately located skin crease, usually 1 to 2 cm inferior to the angle of the jaw. This incision provides excellent cosmesis postoperatively; frequently, the resulting scar is all but invisible. However, if the incision is made in a suboptimal location, more cephalic and caudal exposure in the wound is difficult to obtain. Therefore, if the surgeon is not experienced with a skin crease incision, it is best to make a skin crease incision on the basis of the location of the carotid bifurcation, known either from preoperative DSA, CTA, or MRA or from intraoperative DUS examination, or to use a longitudinal incision. With experience the surgeon will become more comfortable making the incision according to the location of the carotid pulse, although this can be misleading at times. If the incision is made too low, more cephalic exposure can be obtained by extending the skin crease incision posteriorly. If the incision is made too high, more caudal exposure can be obtained by extending the incision more anteriorly.
Carotid Exposure
A meticulous and hemostatic surgical technique is paramount for a successful operation. Manipulation of the carotid artery should be minimized because intraoperative embolization can result from careless handling.
The surgeon begins the dissection by dividing the platysma and mobilizing the medial border of the sternocleidomastoid muscle. The external jugular vein lies deep to the platysma and should be sought in this plane to avoid injury or to retrieve it if it is needed for patching. It is more commonly encountered with an oblique skin crease incision than with a longitudinal incision. The other structure located at this level is the greater auricular nerve; injury to this nerve leads to numbness of the earlobe, which is bothersome to patients, especially those who wear earrings (see under “Cutaneous Sensory Nerves”). The medial border of the sternocleidomastoid muscle is mobilized and retracted laterally with self-retaining retractors. The carotid sheath is entered and the medial border of the jugular vein dissected. The facial vein is identified crossing medially in the base of the wound and divided; sometimes it has an early bifurcation or trifurcation, and multiple branches need to be ligated. The jugular vein is then retracted laterally. The vagus nerve is identified at this point in the carotid sheath, usually located posteriorly between the jugular vein and carotid artery, although in a minority of patients it may lie anteriorly.
Dissection is continued down onto the distal CCA, which is controlled circumferentially with an umbilical tape and a Rumel tourniquet. At this point the ansa cervicalis nerve should be identified; it usually lies medial to the distal CCA. Identifying this nerve facilitates safe dissection of the carotid bifurcation and avoids injury to the hypoglossal nerve, which crosses medially from a superior to an inferior location. The surgeon dissects superiorly along the ansa hypoglossi and continues dissection along the posterior edge of this nerve. As he or she follows the nerve cephalad and encounters its junction with the hypoglossal nerve, the surgeon continues the dissection safely along the posterior border of the hypoglossal nerve with minimal risk of injury; if the surgeon were to dissect along the anterior border of the ansa, it is possible to inadvertently transect the hypoglossal nerve in the crotch of the junction of these two nerves before the hypoglossal nerve is identified (Fig. 100-2). It is important to note that the position of the hypoglossal nerve can be quite variable. Often it lies sufficiently cephalad and medial that it may not be in the operative field. In most cases, however, it crosses the ICA in a position where it may need to be mobilized by division of the sternocleidomastoid branch of the occipital artery, which tethers the nerve (see Figure 100-10).
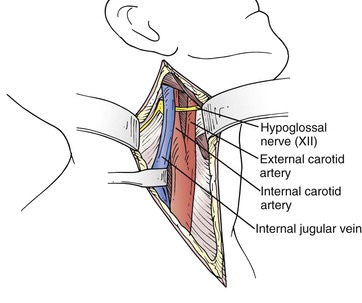
Figure 100-2 Operative field. Note the internal jugular vein mobilized posteriorly after ligation of the anterior facial branch and the hypoglossal nerve crossing the vessels superior to the bifurcation.
At this point the carotid bifurcation is carefully exposed. The superior thyroid artery is identified coming off the medial border of the carotid bifurcation or proximal ECA and controlled with a tie or plastic vessel loop. Dissection continues cephalad on the medial edge of the bifurcation until the origin of the ECA is identified. It is exposed and controlled circumferentially with a vessel loop. Finally, the ICA is exposed coming off the lateral side of the bifurcation. Extreme care must be taken during this part of the dissection. The artery should be controlled in a location that is above the plaque and completely free of disease; in this location the artery has a typical bluish appearance because of translucency of the vessel. During dissection of the carotid bifurcation and its branches, one should avoid dissecting in the crotch of the carotid bifurcation to avoid injuring the carotid body because such dissection can result in hemodynamic instability and troublesome bleeding. If hemodynamic instability results, the carotid body can be gently injected with 1% lidocaine.
Before clamping, the patient is administered 70 to 100 U/kg of heparin, which is allowed to circulate for 3 minutes. The ICA is clamped first to prevent the embolization that can result when the CCA or ECA is clamped. Care should be taken to make sure that the ICA is clamped on a normal portion of the artery distal to the plaque.
If LA or intraoperative electroencephalography is used for selective shunting, a test clamp on the distal ICA should be applied for at least 3 minutes to check for changes in neurologic responses or electroencephalography (EEG) pattern. If such changes occur, the artery should be unclamped to allow reperfusion before the carotid bifurcation is reclamped and opened; opening the bifurcation and placing a shunt may take 2 to 3 minutes and should not be performed while the brain is already ischemic. However, unclamping the ICA introduces the potential for embolization from disrupted plaque.
If carotid stump pressure is to be measured, clamps are placed on the CCA and ECA, and a needle connected to a pressure line is placed into the distal CCA below the carotid bifurcation. Both clamping the CCA and placing the needle into the artery introduce the potential for embolization.
Conventional Endarterectomy
The conventional technique for CEA consists of a vertical arteriotomy and closure by patch angioplasty. In this case a vertical arteriotomy is begun on the CCA and continued through the carotid bifurcation into the ICA. One should avoid making the incision too close to the flow divider at the ECA origin because doing so could distort the anatomy and make the closure more difficult. If a shunt is used, it is placed in the distal ICA and back-bled before its proximal end is placed into the CCA. Two commonly used shunts are the Pruitt-Inahara and Javid shunts. A third shunt that we prefer is a simple vinyl tube, originally described by Collins et al84 (Fig. 100-3). Because this shunt lies entirely within the artery, it allows the surgeon to almost completely finish closing the arteriotomy before the shunt is removed. Its small diameter permits atraumatic placement in even small ICAs, whereas its short length offers less resistance to blood flow such that physiologic flow in the ICA is maintained. Wilkinson et al85 compared the Pruitt-Inahara and Javid shunts in a randomized trial. Using TCD, they found that the Pruitt shunt was less likely to maintain physiologic flow in the MCA, whereas the Javid shunt was associated with a higher incidence of cerebral embolism at declamping.
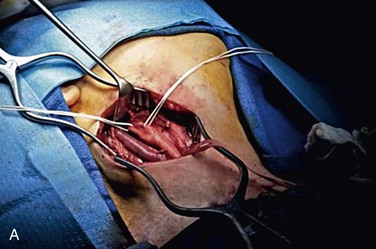
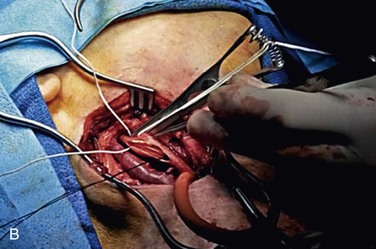
Figure 100-3 A, Initial operative exposure with the Rumel tourniquet around the common carotid artery and the internal and external carotid arteries isolated with vessel loops. B, The arteriotomy has been made and the shunt, with a silk suture attached, lies within the vessel.
The endarterectomy is begun in the CCA in the plane between the media and the adventitia. The proximal end point in the distal CCA is established, and the plaque is trimmed in that location in a beveled manner. The endarterectomy is continued into the orifice of the ECA, first with a Freer elevator and then with a fine clamp that is passed up into the ECA in the plane of the endarterectomy. The clamp is spread apart to further mobilize the plaque away from the adventitia in the 6-, 9-, and 12-o’clock positions; it is usually hard to pass the clamp in this plane at the 3-o’clock position next to the flow divider. The vessel loop on the ECA is released transiently while the plaque is everted from within the ECA. The end point of the plaque is inspected; an ideal end point is gradually tapering and feathered (Fig. 100-4). In our practice, all loose bits of intima and media in the orifice of the ECA are removed to perform complete endarterectomy of the ECA. However, Ascher et al86 have found in a large series that endarterectomy of the ECA may be neglected without compromise of results.86
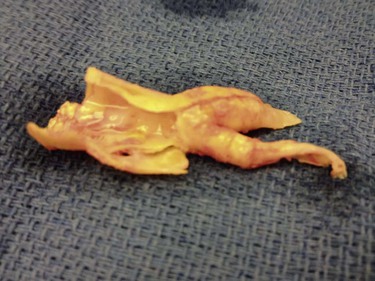
Figure 100-4 Carotid endarterectomy specimen. Note the smooth end points of the distal plaque from the external and internal (longer portion) arteries.
The endarterectomy is continued up into the ICA. A technically perfect end point in the ICA is critical to avoid perioperative stroke and recurrent stenosis. In our experience, it is virtually always possible to achieve a satisfactory end point in the ICA, although special maneuvers may be required to expose the distal ICA and make an extended arteriotomy in this vessel to facilitate extraction of a long endarterectomy specimen, as seen in Figure 100-5. Tacking sutures at the distal end point should be avoided unless absolutely necessary; such sutures are problematic and associated with an increased perioperative stroke rate.87 The endarterectomy should be terminated in normal ICA with a gradual, tapered transition to normal intima; this is best accomplished by pulling the plaque transversely away from the artery with lateral traction. One should avoid pulling out or down on the plaque, which would be more likely to result in a step-off that can be difficult to correct without traumatizing the artery.
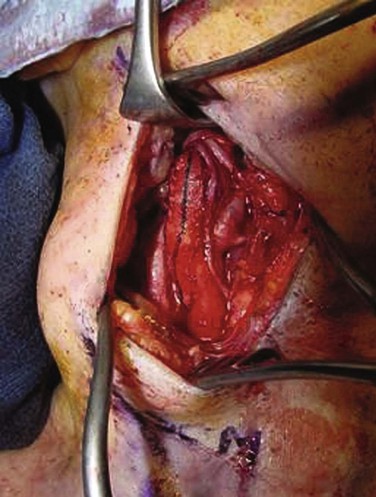
Figure 100-5 Patch closure of the arteriotomy for carotid endarterectomy. Woven polyester (Dacron) was used in this case. Note the ansa cervicalis coursing along the vessel, the vagus nerve at posterior, and the hypoglossal nerve at the apex of the incision.
We believe that repairing the arteriotomy with a patch angioplasty represents the standard of care in contemporary practice (see Fig. 100-4). The patch is sewn in with running nonabsorbable suture. A variety of patch materials are available for use, including autologous vein, polytetrafluoroethylene (PTFE), woven polyester (Dacron), and bovine pericardium. Some studies have suggested that autologous vein may be superior to synthetic patches, but of the prosthetic patches, no material appears to be clearly superior to another (see “Patch Closure”).
Options for autologous vein include the external jugular and saphenous veins. The external jugular vein can be harvested through the same surgical incision and is generally used as a double-layer patch after the surgeon inverts an intact tubular segment of vein without filleting open the vein. Care must be taken to keep the inverted tube flattened out as a rectangular patch while it is being sewn onto the artery. If this is not done, the edges can sometimes roll over or under and lead to a tapered, asymmetric, or severely deformed patch. It is our practice to always start the suture line at the superior end of the arteriotomy in the ICA, which is typically the most difficult—and critical—part of an anastomosis. As the patch material is sewn to one side of the artery, the artery must be stretched out with gentle tension so that an appropriate length of patch is used before it is trimmed; otherwise, there may not be enough patch material available to sew to the other side of the artery in the arteriotomy. When the suture line is nearly completed, the CCA and ICA are reclamped and the shunt is removed. Both clamps are briefly released to flush air or debris (or both) out of the arteries. The clamps should be placed proximal and distal to the patch or endarterectomized surface of the artery because these surfaces can be thrombogenic. The carotid bifurcation is flushed vigorously with heparinized saline and inspected again for debris or intimal flaps before the arteriotomy is finally closed. Once again the clamp on the ICA is briefly released to fill the bifurcation with blood. It is then replaced while the clamps on the CCA and ECA are released so that any remaining air or debris will be flushed up the territory of the ECA rather than the ICA. At this point the ICA clamp is removed.
Any bleeding from the suture line is addressed at this time. However, a final important technical point in this step again relates to the thrombogenicity of stagnant blood in contact with the patch material or endarterectomized surface of the carotid bifurcation. One should avoid reclamping unless absolutely necessary to control bleeding at this stage and avoid the risk of formation of thrombus or a fibrin-platelet aggregate on the patch or endarterectomized vessel. This phenomenon should not be underestimated; in 2002, AbuRahma et al88 noted a 5% carotid thrombosis rate with Dacron patches in a prospective trial with a resultant 7% perioperative stroke rate.88 As a result of that trial, the makers of the Dacron patch reengineered the patch to make it less thrombogenic.
Eversion Endarterectomy
Eversion endarterectomy is an excellent alternative technique that is utilized in many centers throughout the world. Two different versions of eversion endarterectomy are performed. DeBakey et al2 originally described eversion endarterectomy with partial transection of the anterior portion of the carotid bifurcation. Etheredge89 improved on DeBakey’s technique with complete transection of the bifurcation, which allowed the origins of both the ICA and ECA to be everted for a longer distance. The endarterectomy is performed by mobilizing the entire circumference of the carotid adventitia off the plaque (described as a “circumcision” by Etheredge) and then everting the adventitia and mobilizing it upward while gentle caudad traction is applied to the plaque. This maneuver is performed distally into the orifices of the ICA and ECA and then proximally into the CCA. Once the endarterectomy is complete, the divided bifurcation is reunited with a simple end-to-end anastomosis.
Advantages of this technique are that the anastomosis can be performed rapidly and that it is not prone to restenosis, and therefore patching is not required. Disadvantages of this technique are that more extensive dissection is sometimes necessary to mobilize the vessels during the eversion, the procedure does not lend itself readily to shunting (although shunting is not precluded by this technique), and it can be difficult to visualize the end point in the ICA after the plaque has been removed—the artery tends to retract as soon as the plaque pulls away from the adventitia, and it can be difficult to expose and re-inspect this area of the artery again. Therefore, in our opinion, a completion study should be performed with this technique.
In 1985, Kieny et al90 introduced a modification of eversion endarterectomy, in which the origin of the ICA is excised obliquely off the carotid bifurcation, the ICA is inverted on its own, and endarterectomy of the CCA and ECA is performed through an arteriotomy in the side of the carotid bifurcation. With this technique it is easier to manipulate the ICA by itself, so eversion of the ICA is less cumbersome. The ICA is reanastomosed to the carotid bifurcation primarily (Fig. 100-6). This technique allows rapid plaque extraction, the anastomosis is not prone to restenosis, and no prosthetic material is required.
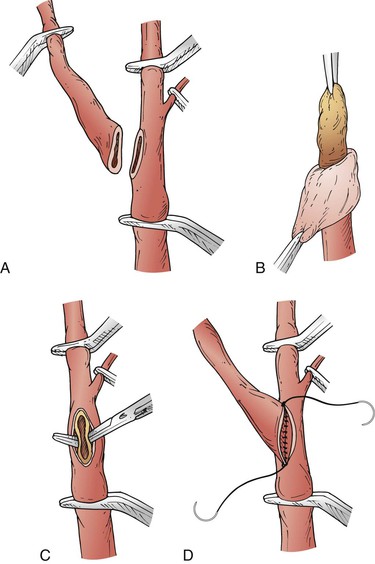
Figure 100-6 Eversion endarterectomy. A, Internal carotid artery transected from the bifurcation. B, Adventitia teased back off the internal carotid artery plaque. C, Plaque endarterectomized from the common carotid artery and origin of the external carotid artery. D, Reanastomosis of the internal carotid artery to the bifurcation. (From Saratzis N, et al: Eversion carotid endarterectomy illustrated: tips and tricks of the procedure. Surg Rounds August 2006. Available at http://www.hcplive.com/general/publications/surgical-rounds.)
This technique is particularly effective for dealing with a redundant, coiled, or kinked ICA. The ICA can be pulled down and straightened and the redundant portion excised. The remaining portion of the ICA is spatulated and reattached to the arteriotomy on the carotid bifurcation. This procedure typically is not performed with a shunt but does not preclude shunt use. Less dissection is required than with transection of the bifurcation. However, exposure for thorough endarterectomy of the CCA and ECA may be suboptimal.
Comparison of Conventional Carotid Endarterectomy and Eversion Carotid Endarterectomy
Numerous studies have compared standard CEA plus patching with eversion CEA. Perhaps the most important is EVEREST (EVERsion carotid Endarterectomy versus Standard Trial), a randomized prospective multicenter study performed in Italy and published in 1997.91 More than 1400 patients were randomly assigned to eversion or standard CEA, with shunting and patching done at the discretion of the operating surgeon. There were no statistically significant differences in outcomes between the two techniques, although a slightly higher incidence of perioperative complications was noted with eversion CEA and a slightly higher incidence of restenosis with standard CEA. A subsequent publication in 2000, however, reporting longer-term follow-up in EVEREST, demonstrated no significant differences in the clinical outcomes between the two CEA methods. At 4-year follow-up, life-table estimates of the risk of restenosis were 3.5%, 1.7%, and 12.6%, respectively for eversion CEA, conventional CEA with patch, and conventional CEA with primary closure.92 These findings are consistent with another large institutional series from the Massachusetts General Hospital involving 950 patients, in which there was no significant difference in the rate of restenosis between eversion CEA and conventional CEA.93 Although other studies have shown better outcomes with eversion CEA,94,95 a large meta-analysis comparing eversion with conventional CEA found no significant differences in the rate of perioperative stroke or death (1.7% vs. 2.6%, OR 0.44, 95% CI 0.10-1.82), but did note that eversion endarterectomy was associated with a lower rate of restenosis during follow-up (2.5% vs. 5.2%, OR 0.48, 95% CI 0.32-0.72).96 Other studies have shown no difference between the two techniques.97,98 Thus, it seems clear that neither technique is necessarily better than the other. Rather, the surgeon should utilize the technique that he or she is most experienced and comfortable with, with the exception that the eversion technique may be better suited for the patient with an elongated and/or kinked ICA.
Exposure for High Lesions
The carotid bifurcation can be located anywhere between the second and seventh cervical vertebrae, and a bifurcation located high in the neck poses technical challenges that can increase perioperative risk for stroke and cranial nerve injury. Ideally, a high bifurcation will be identified on the preoperative imaging study. This is a potential advantage of CTA, images of which always included bony anatomy. A conscious effort must be made to locate the bony anatomy with DSA if unsubtracted images are not provided, but the anatomy can still be defined from subtracted images. Bony landmarks are never provided with carotid DUS, but an astute vascular technologist will note a high bifurcation and should record it in the report.
Nasotracheal Intubation
Several methods can be used to gain additional cephalic exposure of a high carotid bifurcation. The easiest of them—and the initial approach—is to start the operation with nasotracheal intubation. With the patient’s mouth closed, the vertical ramus of the mandible is displaced anteriorly 1 to 2 cm relative to its position when the mouth is open with an oral endotracheal tube. The additional few millimeters of exposure afforded by this maneuver can often make possible a suitable endarterectomy end point in the distal ICA.
Division of the Digastric Muscle
The next step to enhance distal exposure is to divide the posterior belly of the digastric muscle. This muscle takes the same diagonal course through the wound as the hypoglossal nerve but is located superficial to the nerve. Therefore, this nerve should be carefully identified and protected before the muscle is divided. Two other nerves that can be injured high in the neck are the spinal accessory nerve, which enters the tendinous portion of the sternocleidomastoid muscle, usually in the upper third of the muscle, and the glossopharyngeal nerve, which lies deep to the digastric muscle.
Resection of the Styloid Process
The final maneuver that can be extremely effective in gaining cephalic exposure is resection of the styloid process (Fig. 100-7). After the posterior belly of the digastric muscle is divided, the insertions of the styloid apparatus—styloglossus, stylopharyngeus, and stylohyoid muscles—on the styloid process are excised with a scalpel with either a No. 15 blade or a Beaver blade. The stylohyoid muscle can be found posterosuperior to the ECA and inserts into the posterior portion of the styloid process.99,100 In addition, the occipital artery runs along the inferior border of the posterior belly of the digastric muscle. Identification and division of the occipital artery can prevent bleeding secondary to trauma caused by retraction (see Fig. 100-7). The styloid process is carefully resected with a rongeur.101 This step can extend the exposure of the ICA by an additional 4 to 5 mm. However, damage to the underlying facial nerve is a potential complication of styloidectomy.102 In our experience with four cases in which resection of the styloid process was necessary, this maneuver alone permitted exposure of the ICA all the way to the skull base.
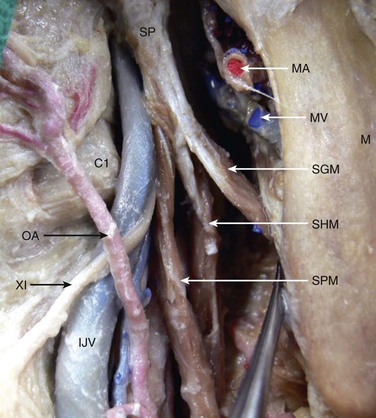
Figure 100-7 Anatomic relationships of the styloid process. C1, Transverse process of first cervical vertebra; IJV, internal jugular vein; M, mandible; MA, maxillary artery; MV, maxillary vein; OA, occipital artery; SGM, styloglossus muscle; SHM, stylohyoid muscle; SP, styloid process; SPM, stylopharyngeus muscle; XI, spinal accessory nerve. (From Izci Y, et al: Retromandibular fossa approach to the high cervical internal carotid artery: an anatomic study. Neurosurgery 62(Suppl 2):ONS363-ONS370, 2008.)
Anterior Subluxation of the Mandible
Two other options can be used to improve exposure of a high bifurcation, and both require preoperative planning and coordination with an oral or plastic surgeon (Fig. 100-8). The patient should receive nasotracheal intubation. Simonian et al103 reported on anterior subluxation of the mandible for use in high carotid exposures in 1984. They described their technique for subluxation as an evolution from full-mouth arch bars with circumdentate wiring to unilateral subluxation by circummandibular/transnasal wiring.103 For the patient with healthy dentition, anterior subluxation of the mandible can be accomplished by placing circumdental wires around the mandibular cuspid and bicuspid teeth on the side ipsilateral to the vascular lesion. Corresponding wires are placed around the corresponding contralateral maxillary teeth. The mandible is then subluxed anteriorly, and the wires are twisted together to hold the fixation. In the patient with poor dentition arch bars can be placed prior to wiring to facilitate placement of the wires.104 An even more aggressive approach involves the use of a complete vertical osteotomy through the vertical ramus of the mandible and separation of the mandible to expose the ICA.
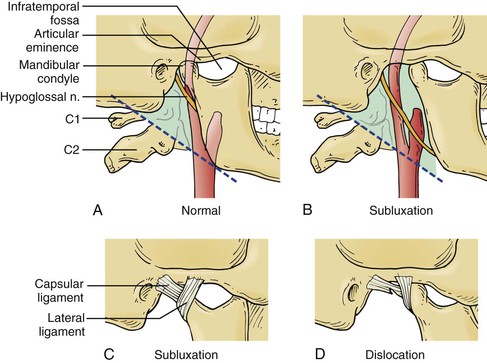
Figure 100-8 Mandibular subluxation/dislocation. A, Normal bony anatomy with high carotid bifurcation. B, The mandibular condyle is pulled forward onto the articular eminence, providing additional exposure when the subluxed mandible is fixed in place at this position. C, Illustrates the desired position of the temporomandibular ligaments during subluxation. D, Illustrates the potential undesired position of the temporomandibular ligaments from dislocation, which may cause a tear in the capsular ligament. (Reproduced from: Simonian GT, et al: Mandibular subluxation for distal internal carotid exposure: technical considerations. J Vasc Surg 30:1116-1120, 1999.)
Cerebral Protection and Monitoring
Shunting
One of the issues of long-standing debate related to the performance of CEA concerns the use of intravascular shunts: routine nonuse of shunts, selective use of shunts, and routine use of shunts. The simplest way to perform CEA is to just clamp the carotid bifurcation and perform CEA without a shunt, and several large series have documented excellent results of CEA without shunts.105–107 However, all these studies report at least a small incidence of stoke, and in at least some cases the etiology of the stroke is intraoperative cerebral ischemia during carotid artery clamping.
Alternatively, some surgeons routinely use shunts in all cases of CEA, and excellent results have been reported in several large series with this practice.108–111 However, all these studies document an incidence of stroke, and in some of these cases the cause of the stroke was attributed to technical problems related to use of the shunt.
A third option, founded on the notion that the use of a shunt conveys its own potential risks, is to use shunts selectively in the patient who would be at high risk for ischemic stroke if a shunt were not used. However, accurate identification of the small group of patients who require shunts has been difficult. Several techniques have been used to identify patients who truly need a shunt. In a patient under GA, such techniques include intraoperative measurement of carotid “stump pressure” after the CCA and ECA have been clamped, intraoperative neurologic monitoring via EEG or somatosensory evoked potentials (SSEPs), measurement of MCA flow by TCD, and monitoring with cerebral oximetry. An alternative method is to perform CEA with the patient under RA, with selection of patients for shunting based on alterations in the neurologic responses that develop after the carotid artery is clamped.
The etiology of intraoperative stroke may be ischemic or embolic. Ischemic stroke results solely from inadequate cerebral perfusion related to clamping of the carotid artery. Embolic stroke can occur during manipulation of the carotid artery before clamping, during the actual process of clamping of the carotid artery, or during reperfusion—either after placement of a shunt or after unclamping of the carotid bifurcation as the endarterectomy is completed. Embolic stroke can also occur as a result either of technical defects in the endarterectomy site or adjacent vessels related to damage from a clamp or a shunt or of a technically inadequate endarterectomy. This latter situation can lead to intraoperative stroke or (delayed) postoperative stroke. All the adjuncts described in the preceding paragraph are designed to detect intraoperative cerebral ischemia, whereas TCD has the added advantage of detecting intraoperative emboli.
Placement of a shunt has the capacity only to prevent ischemic stroke, but it could actually increase the risk for embolic stroke if performed poorly. Intraoperative cerebral ischemia is a relatively uncommon cause of intraoperative stroke.112–114 This fact would support the argument of the “routine nonshunters,” who believe that cerebral ischemia is a rare cause of stroke and that shunting may do more harm than good (by causing embolic complications) such that even selective shunting is never justified. However, there is no denying that when cerebral ischemia does occur, it can lead to perioperative stroke.115–117
It is clear that cerebral ischemia can be completely relieved by placement of a shunt. This should translate into prevention of stroke in the patient if embolic complications related to shunt placement are minimized. In fact, there is evidence that the benefits of shunting outweigh the risks in patients with cerebral ischemia.118–120
A meta-analysis by AbuRahma et al104 looked at all papers published on CEA from 1990 through 2010 and examined the perioperative outcome of routine shunting, routine nonshunting, and selective shunting. The mean perioperative stroke rate was 1.4% for CEA with routine shunting and 2%. for CEA with routine nonshunting. For selective shunting, the stroke rate varied depending upon the method used for intraoperative monitoring. It ranged from 1.1% for cervical block anesthesia, to 1.6% for EEG and carotid stump pressure, to 4.8% for TCD. The investigators concluded that both routine shunting and selective shunting are acceptable and should be employed at the surgeon’s discretion.104
From a medical-legal point of view, routine nonshunting with use of GA would be hard to defend in the case of a patient who suffered an intraoperative stroke during CEA. Consequently, routine nonuse of shunts cannot be advocated, although it is still practiced by those who favor eversion endarterectomy,121 in which placement of a shunt is technically more difficult but not impossible.
Cerebral Monitoring
Selective shunting depends on using a technique to identify patients with intraoperative ischemia. The majority of CEAs are performed today with GA, and strategies to assess cerebral ischemia include stump pressure measurement; EEG, TCD, and SSEP monitoring; and measurement of regional blood and cerebral oxygen saturation. The problem is that none of these techniques is completely accurate. They do not uniformly predict the occurrence of intraoperative ischemia, nor do they prevent the unnecessary use of shunts. Finally, the selective use of shunting may actually increase the risk for an ischemic stroke that would not otherwise occur with routine shunting.
Stump Pressure
Measurement of carotid stump pressure was the first method used to predict intraoperative ischemia.122 In patients with measured stump pressures lower than 50 mm Hg, Hays et al123 noted a 50% neurologic event rate in those who were not shunted versus a 10% rate in those who were in a series of 297 patients.
With the advent of other adjuncts to detect cerebral ischemia, such as intraoperative EEG and TCD, studies were performed to validate carotid stump pressure measurement. Kelly et al124 measured stump pressure with concurrent EEG monitoring in 289 patients and performed shunting only in those with evidence of ischemia by EEG criteria. They found that 6% of patients with stump pressure higher than 50 mm Hg had ischemia by EEG criteria. Stump pressure correlated well with EEG findings in patients with completed strokes but had a false-negative rate of 77% in patients with vertebral artery disease. In another study using electroencephalography as a “gold standard,” Harada et al125 found that a stump pressure lower than 50 mm Hg had a positive predictive value of only 36%. In this study, 11% of patients with ischemia by EEG criteria would not have received shunts, and 64% of patients with a stump pressure lower than 50 mm Hg would have received shunts unnecessarily if determined only by stump pressure criteria. Similarly, Brewster et al126 found that 11 of 17 patients with ischemic EEG findings would not have received shunts if determined only by stump pressure criteria, and 7 of 63 would have received unnecessary shunts. Finocchi et al127 used TCD to verify stump pressure and found that stump pressure did not correlate well with ischemia by TCD criteria in patients with postoperative deficits. Clearly, even in the setting of what appears to be a satisfactorily high stump pressure, there may still be regions of the brain that are relatively hypoperfused.
Electroencephalographic and Somatosensory Evoked Potential Monitoring
Intraoperative EEG monitoring is the most widely used method of intraoperative cerebral monitoring. It can be performed with 8, 12, or 16 leads, the 16-lead configuration being standard. Standard criteria for intraoperative ischemia are at least a 50% decrease in fast background activity, an increase in delta wave activity, or complete loss of EEG signals. With use of these criteria, shunt use can be minimized.128,129 In some of the studies of this issue, the decreased use of shunts resulted in improved neurologic outcome,130,131 whereas in others, neurologic outcome was unchanged or even worse.128,129 A common finding in all the studies is that EEG is overly sensitive—positive in 10% to 40% of patients with unilateral carotid disease and positive in as many as 69% with bilateral carotid disease,132,133 thereby overestimating the number of people who require shunts. Blume et al134 observed postoperative strokes in only 9% of patients with abnormal EEG findings in whom shunts were not placed.134
Furthermore, several series have documented neurologic events that occurred in the absence of EEG abnormality when shunting was not used.129,135 Tempelhoff et al135 found that five of six patients with postoperative deficits in a series of 103 patients showed EEG changes only late in the operation, when shunting was no longer feasible.
Similarly, there are multiple published studies on the use of SSEP suggesting that it is a useful adjunct to detect ischemia.136–140 However, in a meta-analysis of 15 studies, Wober et al141 found that SSEP monitoring is not a reliable means of detecting ischemia and predicting neurologic outcome.
Transcranial Doppler Ultrasonography
The use of TCD during CEA was introduced by Schneider et al142 in 1988. Visser et al143 reported that one could safely avoid shunting in a third of patients with normal TCD findings but that abnormal findings on TCD predicted ischemia by EEG criteria only 60% of the time. TCD has the unique advantage of detecting microemboli intraoperatively, possibly alerting the surgeon to avoid further manipulation that may cause a stroke.144,145 However, Belardi et al146 also reported that TCD (as well as stump pressure) was not accurate in predicting cerebral ischemia.
Awake Carotid Endarterectomy with Regional or Local Anesthesia
In view of these findings, performing CEA with RA is the most reliable method of predicting the need for selective shunting. It can be done with either a cervical plexus block or purely local anesthesia. In this case the decision to place a shunt is based solely on the development of hemispheric or global neurologic symptoms after the carotid artery is clamped. This criterion is considered the standard for selective shunting to which all other adjuncts should be compared. Shunt rates with this approach, on the order of 5% to 15%, are consistently lower than with other modalities.146–153 In two prospective trials in which both stump pressure and EEG measurements were recorded before endarterectomy with RA but in which the need to shunt was ultimately determined by neurologic changes, both EEG findings and stump pressure were found to inaccurately predict the need for shunting.154,155 In the study by Calligaro et al,154 published in 2005, a cost analysis found that RA saved more than $3000 per case by avoiding EEG measurements.
In addition to its benefit in predicting the need for shunting, other potential benefits of performing CEA on awake patients have been suggested. One study has demonstrated a lower rate of MI with RA that was statistically significant,156 whereas others have not confirmed this benefit.151,157,158 Bowyer et al158 showed a statistically significant decrease in the neurologic event rate with RA, but not all patients were selectively shunted in this study, and no other study has duplicated this finding. Three studies have shown decreased length of stay with RA,150,156,157 but in two of them the length of stay, approximately 5 days, was not reflective of modern practice.150,157 This decrease does not necessarily translate into reduced cost,159 but RA is probably the only adjunct for selective shunting that does not increase cost over that of routine shunting. Finally, as mentioned earlier for the multicenter randomized GALA trial, the definitive study of RA versus GA in CEA that included more than 3500 patients, there was no significant difference in the rates of perioperative stroke, death, or MI between the two anesthetic methods. There was, however, a higher rate of perioperative stroke among patients with contralateral occlusion who underwent CEA under general GA, although this difference was not statistically significant.81 Furthermore, in a post-hoc analysis of the GALA data, costs were less within the first 30 days after surgery in the RA patient cohort.160
The disadvantages of RA are that not all anesthesiologists, surgeons, or patients are comfortable with performing CEA with use of RA. Cervical block is an advanced RA technique that requires considerable skill on the part of the anesthesiologist. Many surgeons find it stressful to have an awake patient who can behave unpredictably during the procedure. Some patients tolerate RA poorly because of claustrophobia or inadequate anesthesia with just a regional or local block. There is also a small risk of seizure or cardiac arrhythmia with inadvertent administration of local anesthetic into the carotid artery or jugular vein.
Routine Shunting
One limitation in the strategy of selective shunting is that with the exception of performing the procedure with RA upon the awake patient, none of the methods to assess the need for a shunt is absolutely accurate. When stump pressure measurement is utilized to determine the need for a shunt, instrumenting the carotid artery with a needle introduces the added risk of embolism. On the other hand, when the operation is performed in the awake patient, the need for shunt placement is determined through the patient’s neurologic reaction to a test clamp. When a test clamp response is positive, there are two options. One is to unclamp the vessels and reperfuse the brain while preparations are made to place the shunt. This maneuver exposes the patient to the risk of embolization. The other option is to proceed with the endarterectomy and placement of a shunt. In this instance, the brain is ischemic when the test clamp response becomes positive and is exposed to an additional period of ischemia while the shunt is being placed, whereas during routine shunting, shunt placement can usually be accomplished in 1 to 2 minutes after clamping, before the brain becomes ischemic. The additional period of ischemia after a positive test clamp response can be detrimental. Tempelhoff et al135 reported that five of six postoperative neurologic deficits occurred in patients who had ischemic times longer than 9 minutes by EEG criteria.1
Although there is no definitive clinical evidence to support one shunting strategy over another,161,162 the routine use of carotid shunts eliminates these concerns. It allows CEA to be performed in a consistent manner, thereby eliminating surgeon and patient anxiety, without the added cost or complexity of monitoring equipment. The shunt is easy to place and facilitates performing CEA in a teaching environment. All studies consistently show that carotid shunting with either the Javid or Pruitt-Inahara shunt completely relieves the intracerebral ischemia caused by clamping, whether measured by neurologic status, EEG changes, MCA flow on TCD, or cerebral blood flow. The placement of a shunt introduces the risk of iatrogenic complications such as intimal dissection as well as of thromboembolic complications, but in the hands of experienced operators, the procedure is very safe. An absolute requirement for safe shunt placement is that the superior end of the plaque be positively identified and adequately exposed through the arteriotomy so that the distal end of the shunt does not “snowplow” into the plaque when it is placed, causing embolization or dissection. There is evidence that placing a shunt in the setting of severe ischemia decreases the stroke rate.162 As noted earlier, minimizing ischemic time in the brain by routine shunt placement has the theoretical advantage of limiting ischemia-reperfusion injury.
Pärsson et al163 have shown that carotid shunting diminishes the inflammatory response of ischemic brain injury, as demonstrated by the production of various inflammatory mediators. This may be an important mechanism in the occurrence of delayed postoperative strokes, which can account for up to 70% of perioperative strokes.113 Numerous large series document excellent results with the use of routine shunting in CEA. Thompson109 demonstrated superb results over a 15-year period spanning the 1960s and 1970s, with a stroke rate of 1.4% in 1107 CEAs. Javid et al110 reported similarly excellent results in a series of more than 1800 patients from the same era. In 1997, Hertzer et al111 reported a series of more than 1900 CEAs performed at the Cleveland Clinic, virtually all of which involved routine shunt placement, with a perioperative stroke rate of 1.8%.111 Hamdan et al108 published a series of 1001 consecutive patients in whom routine shunting was used most of the time and in whom the combined stroke and death rate was 1.6%. Clearly, there are other reports documenting similarly outstanding results among patients undergoing CEA with selective shunting,164 although selective shunting may somewhat increase the complexity of the procedure and the associated costs.
In summary, there is no definitive evidence to support the superiority of one shunting strategy over the others. As noted previously, a meta–analysis has documented a mean perioperative stroke rate 1.4% for CEA with routine shunting and of 2% for CEA without routine shunting and from 1.1% to 4.8% for selective shunting, depending on the monitoring method.165 Finally, despite acceptable results in some individuals’ hands, routine nonuse of shunts cannot be advocated in view of the abundant evidence that intraoperative ischemia can be a source of stroke that is preventable with shunts.
Arteriotomy Closure
When CEA is performed through a longitudinal arteriotomy, an important consideration is how the arteriotomy is closed. It has been known for some time that primary closure of a longitudinal arteriotomy, which is the simplest and most efficient way to close an arteriotomy, can result in significant stenosis of the vessel. However, considerable experience and level I evidence indicate that patch closure yields superior clinical and anatomic outcomes.
Patch Closure
Saphenous Vein Patches
Before the advent of synthetic patches, the surgeon’s choices for patch material were saphenous vein and external jugular vein. Imparato used vein patching routinely as early as 1965, but no rigorous comparison of the results of patch angioplasty and direct closure was made until more than 20 years later.166 Saphenous vein patching has been used extensively with good results, although problems specific to saphenous vein patching include wound complications at the harvest site, potential compromise of a valuable conduit for later bypass procedures, and the devastating complication of patch rupture, which has been reported to occur in 0.5% to 4% of cases.167–171 Because most of these ruptures were associated with ankle veins, several investigators recommended harvesting the great saphenous vein (GSV) from above the knee. Lord et al172 also noted that aneurysmal expansion of saphenous vein patches can occur in up to 17% of patients.
Archie et al173 investigated the relationship of GSV diameter and rupture pressure and found that GSVs with diameters less than 3.5 mm were more prone to rupture. Their group also noted that women were three times more likely to have GSVs measuring less than 3.5 mm. Applying this knowledge to their practice, Archie174 found that using a GSV with a distended vein diameter of more than 3.5 mm and maintaining a carotid bulb diameter of less than 13 mm avoided patch rupture completely in a series of 534 patients over an 8-year period.
Synthetic Patches
As synthetic patch materials became available, they were incorporated into practice to avoid the pitfalls of saphenous vein patching. Synthetic materials that are commonly used for this purpose are polytetrafluoroethylene (PTFE), woven polyester (Dacron), and bovine pericardium. Studies on the influence of the type of carotid closure have therefore not only compared carotid patching with primary closure but also analyzed outcomes with different patch materials.
Comparative Analyses
The British Joint Vascular Research Group RCT compared 104 patients undergoing primary closure with 109 patients treated by patch closure with either autologous vein or Dacron.175 Six strokes occurred in the primary closure group and two in the patch group, and six perioperative thromboses were noted in the primary closure group versus none in the patched group. At 1 year there were 17 occlusions or greater than 50% stenoses in the primary closure group versus 6 in the patch group. In 1994, Katz et al,175 reporting on 100 patients undergoing CEA who were randomly allocated to undergo primary closure (n = 51) or patch closure with PTFE (n = 49), found no significant difference in outcomes, although there was a trend toward higher neurologic morbidity in the primary closure group. AbuRahma et al176 randomly assigned 74 patients with bilateral carotid stenoses to primary closure followed by patch closure or patch closure followed by primary closure to eliminate systemic factors that might confound results in different patients, such as female gender, smoking, hyperlipidemia, young age, diabetes mellitus, hypertension, and disseminated atherosclerosis. They found a significantly higher perioperative stroke rate for primary closure than for patch closure (4% vs. 0%), as well as a higher stroke/TIA rate for primary closure than for patch closure (12% vs. 1%). All the strokes in the primary closure group were due to perioperative thrombosis of the carotid bifurcation. With long-term follow-up to 5 years, there was also a statistically significant difference in the rate of repeat carotid surgery, 14% in the primary closure group versus 1% in the patch closure group.
Pooled data from meta-analyses and large databases unequivocally support the use of patch closure over primary closure. Meta-analyses by Counsell et al177 in 1997 and Bond et al178 in 2004 found that all short-term (30-day) and long-term end points were significantly better with patch closure than with primary closure. Comparison of vein patches with prosthetic patches in these studies demonstrated no significant difference in the major end points.177,178 Rockman et al179 used a state database in New York to review outcomes with primary closure in comparison with patch angioplasty or eversion endarterectomy in 1972 CEAs at six regional hospitals. Perioperative stroke was significantly more common with primary closure than with eversion or patch closure (5.6% vs. 2.2%, no difference between eversion and patching), as well as higher perioperative stroke rates and death with primary closure than with eversion or patch closure (6% vs. 2.5%). There were no differences in any outcomes between eversion and patch closure. Finally, Kresowik et al180 reported the outcomes of more than 10,000 CEAs performed in several states and found that use of a patch, in particular a prosthetic patch, was a statistically significant indicator for improved outcomes. On the basis of these findings, the U.S. Centers for Medicare and Medicaid Services adopted patch closure of conventional CEA as a physician quality measure for 2009.
After these studies were published, Al-Rawi et al181 reported no differences between primary closure and closure with a collagen-coated polyester patch in a fairly large RCT that included 328 patients. Of note, primary closure was performed by a single surgeon using microvascular techniques. There were no statistically significant differences in outcomes, but a trend toward a higher combined stroke and death rate was noted in the patch group.181 Therefore, primary closure with microvascular technique may be the only instance in which it might be justified. Otherwise, the preponderance of evidence supports patch closure after conventional CEA.
Optimal Patch Material
The optimal patch material remains to be defined. Grego et al182 compared the results of patch closure with external jugular vein and with PTFE in a randomized trial of 160 CEAs and reported no difference in stroke-free survival at 12, 30, and 60 months and no difference in recurrent stenosis rates, but a trend toward better results with vein patch. Marien et al183 performed an RCT in which bovine pericardium was compared with Dacron in 95 CEAs performed in 92 patients and observed significantly less suture line bleeding with bovine pericardium than with Dacron (4% vs. 30%) but no difference in neurologic outcomes. Goldman et al184 compared saphenous vein and internal jugular vein with knitted Dacron in 275 CEAs and found no significant differences in perioperative morbidity, mortality, or early restenosis among any of these groups.
In a small trial comparing internal jugular vein, GSV from the thigh, and knitted Dacron, Jacobowitz et al185 found a higher death rate in the jugular vein group (8% vs. 0% with GSV and 1.1% with knitted Dacron) as well as a higher stroke rate that was not statistically significant (4% vs. 1.3% and 1.1%, respectively).185 AbuRahma et al186 examined the outcomes of primary closure in comparison with patch closures using GSV, jugular vein, and PTFE. They found that perioperative neurologic event rates were significantly higher with primary closure than with all patch methods. Event rates were slightly higher with the jugular vein patch, and recurrent stenosis was also higher with this patch than with GSV or Dacron patch and similar to those for primary closure. In a follow-up study 2 years later, the same investigators reported that primary closure had a higher incidence of restenosis and need for reoperation, especially in women, who had smaller carotid arteries.187
Finally, in a small RCT comparing Dacron with PTFE patching in CEA, AbuRahma et al188 found a higher stroke rate with Dacron that was largely due to perioperative thrombosis. As a result of this trial the manufacturer of the Dacron patch reengineered it, and AbuRahma’s group studied the new patch in a follow-up trial.189 This investigation showed no difference in outcome between the Dacron and PTFE patches. The cause of the difference in outcomes in the original trial was not clear because as long ago as 1996, Margovsky et al190 had found, in an animal model, that platelet accumulation was not significantly different for gelatin-sealed Dacron and PTFE, whereas platelet accumulation was much lower on vein patches than on either of these prosthetic patches.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree