Chapter 102
Carotid Artery
Dissection and Fibromuscular Dysplasia
Rabih A. Chaer, Peter A. Schneider
Nonatherosclerotic carotid disease is uncommon but may have significant clinical manifestations. Among these, fibromuscular dysplasia (FMD) and spontaneous (ie., nontraumatic) carotid dissection are the subjects of this chapter. FMD is a nonatheromatous proliferative process whose principle pathologic form involves primarily the media and involves long, unbranched segments of medium-sized conduit arteries such as the renal artery and the internal carotid artery (see Chapter 147).1–3 Although FMD is a systemic process of unknown etiology, it is usually described in terms of the artery in which it occurs; its principle clinical manifestations involve the spectrum of arterial obstruction and/or aneurysmal degeneration. Carotid artery dissection and FMD can be related. Although dissection is more often spontaneous or posttraumatic in an otherwise normal-appearing artery, it can occur as a complication of FMD.
Fibromuscular Disease
Epidemiology
FMD has been found on 0.25% to 0.68% of consecutive cerebral arteriograms.4,5 Although the prevalence in the general population is not well known, the incidence of carotid FMD was 0.42% in 3600 patients undergoing cerebral arteriographic examination in one study.6 Many of these examinations were performed for suspected cerebrovascular disease, and thus the true frequency of carotid FMD in the general population is probably lower. In one series of 2000 carotid operations, FMD was the identified pathology in 3.4% of cases.7 In another sizable series of carotid operations, dilatation for FMD was performed in less than 1%.8 Carotid FMD is bilateral in 39% to 86% of reported cases.1,4,9,10 Women predominate in most series, constituting 60% to 90% of patients.1,7,9,11 A patient may have evidence of FMD in a single artery or in multiple vascular beds. Although FMD is most commonly believed to affect the renal arteries and less frequently the carotid and vertebral arteries, data on the first 447 patients in the United States Registry for Fibromuscular Dysplasia shows the extracranial carotid and vertebral arteries to be nearly as frequently involved as the renal arteries, primarily in middle-aged women.12 Carotid FMD is associated with cerebral aneurysms and FMD involving the renal arteries. These associations are detailed later in this chapter and are also discussed in Chapter 147.
Pathogenesis
Arterial fibrodysplasia consists of a heterogeneous group of nonatherosclerotic diseases manifested by both occlusive and aneurysmal morphology. The pathology of FMD is discussed further in Chapter 147. Although the etiology of FMD is not known, several theories have been proposed, including environmental and genetic factors, each with partial supporting evidence (Box 102-1). Complications occurring with carotid FMD include encroachment on the arterial lumen causing reduced perfusion, formation and distal embolization of thrombus, and dissection leading to possible rupture. These complications occur in less than 10% of cases. Progression of carotid FMD is not well defined.2,6,13,14 Most cases of asymptomatic carotid FMD are known to remain clinically silent, and there is no optimal method of noninvasive follow-up of these lesions to reliably grade progression. Among the four types of FMD, the internal carotid artery is most often affected by medial fibroplasia, which results in an arteriographic appearance resembling a “string of beads” (Fig. 102-1), seen in 80% to 95% of the lesions.4,7,11,15 In one large series, medial fibroplasia was found in 89% of cases, with fusiform narrowing in 7% and an eccentric septum-like lesion in 4%.4 The arterial segments involved tend to be more distal than occurs with arteriosclerosis and are located in the middle and distal segments of the extracranial internal carotid artery without any appearance of disease at the carotid bifurcation. The serial stenoses are frequently evident on examination of the external surface of the artery (Fig. 102-2). The artery is often elongated and tortuous, and kinking occurs in approximately 5% of cases (Fig. 102-3). Similar disease of the external carotid artery or the intracerebral arteries is rare.
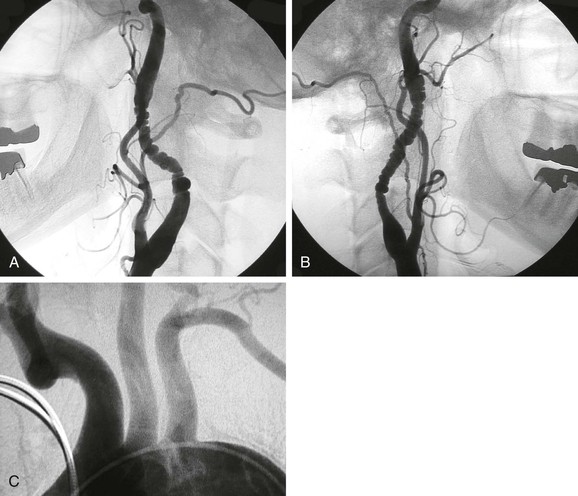
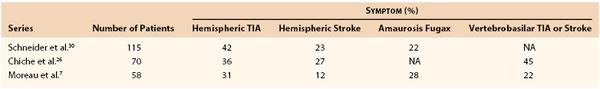
Figure 102-1 A and B, Carotid arteriograms demonstrating the classic appearance of fibromuscular dysplasia in the usual location opposite the C1-C3 vertebral bodies and intervening disks. Note the low bifurcation and long internal carotid artery. The lesions are present bilaterally in this 43-year-old woman. C, The aortic arch shows no evidence of disease.
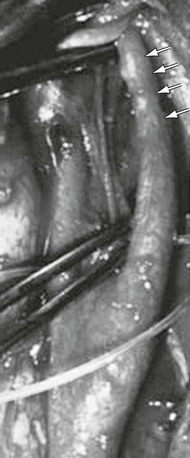
Figure 102-2 Medial fibrodysplasia of the extracranial internal carotid artery. Operative exposure of the artery reveals an external beaded appearance due to serial narrowing.
Figure 102-3 Medial fibrodysplasia of the extracranial internal carotid artery with angulation and stenosis (arrow) affecting a tortuous elongated segment. (From Stanley JC, et al: Extracranial internal carotid and vertebral artery fibrodysplasia. Arch Surg 109:215-222, 1974.)
Concurrent lesions that frequently complicate the management of carotid FMD include (1) atherosclerotic occlusive disease at the carotid bifurcation, (2) extracranial carotid artery aneurysms, (3) carotid artery dissection, (4) vertebral artery FMD, (5) intracranial aneurysms and occlusive disease, and (6) renal artery FMD. In the United States Registry for Fibromuscular Dysplasia, from which data from the first 447 were published in 2012, 342 patients had undergone imaging of the extracranial carotid/vertebral arteries (73% ultrasonography, 30% magnetic resonance angiography, 28% computed tomographic angiography, and 28% catheter-based angiography), and 374 patients imaging of the renal, mesenteric, and/or abdominal aorta. Not all vascular beds were imaged in every patient, so the prevalence of FMD in multiple vascular beds could not be determined, but it was interesting to note that of 357 patients in whom two or more vascular beds were imaged, 126 (35.3%) had FMD in two vascular beds. Of 292 patients in whom three or more vascular beds were imaged, 64 (21.9%) had FMD in three vascular beds. Of 232 patients in whom four or more vascular beds imaged, 21 (9.1%) had FMD in four vascular beds.12
Atherosclerotic Occlusive Disease
Ipsilateral atherosclerosis of the carotid bifurcation is present in as many as 20% of individuals with carotid FMD,7,16 but it may not be possible to attribute cerebral symptoms to one lesion or the other. When atherosclerotic and fibromuscular dysplastic lesions occur together in the same symptomatic artery, they are usually treated simultaneously. In a series of 72 operations in 58 patients for extracranial FMD, carotid endarterectomy was performed as part of a combined procedure in 14 patients (19%).7
Extracranial Carotid Artery Aneurysms
Extracranial carotid artery aneurysms are uncommon, and those associated with FMD are rare. Among 130 extracranial carotid aneurysms in one literature review, 2.3% were associated with FMD.17 In a single-institution series of 15 carotid aneurysms, one third were thought to be caused by or at least associated with FMD, and most were successfully managed with resection and replacement grafting.18 In the U.S. registry for FMD, carotid aneurysms (including the extracranial and intracranial internal carotid artery and the ophthalmic artery) were reported in 16 (21.1%) of 76 patients with carotid FMD.12
Carotid Artery Dissection
FMD may play a role in the development of spontaneous dissection of the carotid artery. This often catastrophic event is responsible for up to 4% of strokes and is associated with fibrodysplasia in approximately 15% of cases.19–21 In a series of 50 patients undergoing surgery for carotid dissection, FMD was the cause in 12% and was treated with either graduated rigid dilatation or vein graft replacement.22 In another series of seven consecutive patients with carotid dissection that was managed by carotid stent placement, FMD was the cause in seven.20 FMD should be considered among the potential etiologic mechanisms of spontaneous carotid dissection.15,23–25 However, reports of spontaneous dissection occurring subsequent to the identification of asymptomatic carotid FMD are not available. In the U.S. registry for FMD, dissection was identified in 19.7% of patients with the disease. The three most common sites for dissection were the carotid arteries (14.8% of all patients enrolled), followed by the renal arteries (4.3%) and the vertebral arteries (3.4%). Carotid artery dissection was prevalent in patients with carotid FMD and was seen in 68 (75.0%) of 88 patients.12 The structural abnormality of the arterial wall in FMD and its association with spontaneous dissection suggest that dilatation of the artery may pose an increased risk for perioperative dissection. Nevertheless, dissection is an unusual complication of dilatation.1,7,26
Vertebral Artery Fibromuscular Dysplasia
Vertebral artery FMD is identified in 7% to 38% of patients with carotid lesions and is occasionally an isolated finding.7,13,26 The vertebral artery disease is usually located at the level of the C2 vertebral body and does not extend intracranially.11 FMD has been associated with vertebral artery dissection after neck or spine manipulation.27 Fortunately, it is rarely responsible for symptoms and does not usually complicate management of the internal carotid lesion. One series reported 32 patients with vertebrobasilar transient ischemic attack (TIA) and stroke as a result of vertebral artery FMD.26 Among 12 vertebral artery reconstructions performed, half were for isolated vertebral FMD and half were performed at the time of rigid dilatation of a carotid lesion. The most commonly used operative approach was a vein bypass to the distal vertebral artery (see Chapter 107).
Intracranial Aneurysms and Occlusive Disease
Intracranial aneurysmal disease is another important expression of the dysplastic process. It is found in at least 10% of patients with FMD in general and as many as 51% of patients with internal carotid FMD in particular (20% on average).9,13 Solitary intracranial aneurysms are present in 80% of these patients, but multiple aneurysms occur in the remaining 20%. In the U.S. registry for FMD, intracranial FMD, manifesting primarily as intracranial aneurysms, was reported in 8.3% of patients.12 These aneurysms tend to be on the same side as the extracranial carotid FMD.9 They pose an independent threat of rupture and hemorrhage, and their natural history has the potential to be worsened by relief of a proximal stenosis. In one series, intracranial aneurysms and extracranial FMD each caused half the symptoms, and in another series, half of the strokes were due to aneurysm rupture.9,28 In most studies, however, intracranial aneurysms were responsible for a quarter to a third of the neurologic symptoms caused by the cervical carotid lesion.2
Nonetheless, intracranial aneurysms should be treated on their own individual merits (e.g., size, initial symptoms). Furthermore, the presence of a small, asymptomatic intracranial aneurysm should not dissuade the vascular surgeon from repairing a significant cervical lesion. The supraclinoid vasculature may also be affected by occlusive disease that suggests FMD as the etiology. This finding is rare and is seen primarily in young and middle-aged women without other apparent etiologic factors. There may be associated evidence of FMD of the cervical internal carotid artery.29
Renal Artery Fibromuscular Dysplasia
Renal artery FMD coexists with carotid FMD in 8% to 40% of patients.2,7,30 It is an additional threat in patients with intracranial aneurysms, in that it is often accompanied by severe hypertension. Because renal artery FMD generally responds well to percutaneous balloon angioplasty, the possibility of coexistent renal artery involvement should be considered before the treatment of carotid stenosis, especially if the patient has hypertension that is difficult to control (see Chapter 147).
Clinical Findings
More than 90% of patients with medial fibroplasia are women, and the disease is diagnosed most often during the fourth and fifth decades of life. Bilateral disease has been reported to occur in 35% to 85% of patients with these lesions. This lesion is rare in African Americans. Although medial fibrodysplasia is considered a systemic arteriopathy, clinically overt arterial involvement is usually limited to the renal, extracranial internal carotid, and external iliac vessels.6,14,26,28
Extracranial cerebral artery FMD may be either an incidental finding without symptoms or the cause of neurologic events. Symptomatic manifestations in large, contemporary series of treated patients included TIA, stroke, and disability (Table 102-1). Stroke was the initial finding in 12% to 27% of patients. Hemispheric TIA occurred in 31% to 42%, and amaurosis fugax was present in 22% to 28%.6,16,17 Several other series have confirmed this distribution of symptoms.13,31,32
Physical examination findings at the time of enrollment were available for 414 patients (92.6%) in the U.S. FMD registry. Findings consistent with Horner’s syndrome (pupil abnormality or ptosis) were reported in 12.4% of patients. Cranial nerve abnormalities were reported in 9.4%, and other focal neurologic deficits in 13.6%. Bruits were reported over the carotid arteries (30.5%; 18.1% bilateral), epigastrium (17.5%), and flanks (6.1%). Among 306 patients with reported imaging of the extracranial circulation (carotid and vertebral) and a documented physical examination for carotid bruits, 227 (74.2%) had FMD. The sensitivity of a carotid bruit for extracranial FMD was 103/227 (45.4%), and the specificity was 74/79 (93.7%).12
Patients can also present with nonspecific symptoms or signs that frequently occur with carotid or vertebral artery FMD, such as dizziness, headache, altered mentation, pulsatile tinnitus, neck pain, headache, wooziness, and a swishing (swooshing, whooshing) sound in the ears. Such symptoms should trigger diagnostic evaluation for carotid FMD, especially in younger patients with no classic risk factors for atherosclerotic disease. Patients can report significant disability because of the constant noise in their ear and they seek support and help with others who are similarly afflicted.33–35
More focal and specific neurologic signs and symptoms may be related to one or more of the following mechanisms: (1) severe stenosis producing hypoperfusion, (2) embolization, (3) thrombosis, (4) dissection, and (5) aneurysm rupture.34,35
Other than this process occurring in a relatively young, mostly female population, the history and physical findings may be notable for the absence of other identifying factors. There may be no particular history or inciting factors. The presence of atherosclerotic risk factors is variable. Physical examination may detect bruits in other locations.
Diagnostic Evaluation
Duplex Ultrasound
Most asymptomatic patients with carotid bruit and those with hemispheric or nonfocal neurologic symptoms undergo carotid duplex ultrasound (DUS) (see Chapter 98). DUS may reveal elevated velocity as a result of FMD, but the lesion may be missed because it is located more distally than the usual atherosclerotic plaque.11,36 If the lesion is detected, the DUS study may not be able to evaluate the artery distal to the lesion because FMD may involve the artery all the way to base of the skull.
Arteriography
Catheter-based contrast-enhanced arteriography continues to be the best method for clearly delineating the anatomic features of FMD of the extracranial cerebral arteries (see “Mechanical Repair”). Many cases of carotid FMD are discovered during arteriography.11 An interesting finding, severe tortuosity in the distal carotid arteries, is seen in patients with FMD. This may occur in the absence of other findings such as beading. However, it is unclear whether this represents another manifestation of FMD.34,35
Computed Tomographic and Magnetic Resonance Angiography
Computed tomographic angiography (CTA) may replace standard catheter-based arteriography in the evaluation of carotid FMD when enough experience has been gained for clinical and anatomic correlation, just as CTA has done in other vascular beds and disease processes. When carotid FMD is identified, the intracranial vascular anatomy should be evaluated to check for the presence of intracranial aneurysms as well as contralateral carotid stenosis and vertebral artery disease. Magnetic resonance angiography (MRA) has not been particularly useful in the diagnostic evaluation of carotid FMD because of the tendency for signal dropout with tight lesions and the propensity to produce a “beaded” appearance in normal conduit arteries that may be confused with FMD. MRA may be beneficial in follow-up of known FMD.37 Computed tomography or magnetic resonance imaging of the brain should be performed in patients with carotid FMD to look for evidence of infarction as well as for cerebral aneurysms.
Selection of Treatments
Natural History
Our understanding of the natural history of FMD of the carotid circulation is not complete. Many series have documented the potential for carotid FMD to cause symptoms, as discussed earlier, but the natural history of asymptomatic lesions is less well documented. In one series of 79 patients, most of whom were found to have carotid FMD incidentally on cerebral angiography (0.6% of the total), only 3 patients (4%) subsequently suffered a cerebral ischemic event during an average follow-up of 5 years.14 When small groups of asymptomatic patients were studied prospectively, less than 10% went on to experience new neurologic symptoms.2,9,14 Roughly a third of carotid FMD lesions demonstrate significant angiographic progression with time.2,13 None of these studies, however, has included a significant number of patients with high-grade asymptomatic stenoses, the group in which the risk for stroke would be expected to be higher. At this juncture, given the favorable behavior of asymptomatic disease, patients with asymptomatic carotid FMD should be monitored and treated medically, with antiplatelet therapy, for primary stroke prevention. There is no evidence to suggest a role for lipid-lowering therapy in patients with isolated FMD, although it should be considered in patients with abnormal low-density lipoprotein cholesterol (LDL) levels and evidence of atherosclerotic occlusive disease as part of a primary prevention strategy.
Most reported cases of symptomatic carotid FMD have been treated with dilatation of the responsible artery. There is no study in which a large number of patients with carotid FMD and focal cerebral ischemic events were treated medically. In one series, 13 patients with either TIA (10 patients) or stroke (3 patients) did not undergo surgical correction of carotid FMD. Only 1 patient remained symptomatic.37 Some have suggested that even symptomatic lesions should not be considered for surgery until the natural history of FMD is better understood.28,38
Another rare potential indication for invasive therapy in patients with carotid FMD includes disabling intractable headaches. Percutaneous interventions have been offered to such patients, although the pathogenesis of headache in such patients is not known. The key is in careful patient selection, reserving invasive therapy for patients with severe disabling symptoms, after other common etiologies have been evaluated and ruled out and after conservative therapies are exhausted.34,35
At present, we are left with a rare cause of focal cerebral ischemic events—extracranial carotid stenosis, which can be repaired with a fairly simple open operation or with percutaneous balloon dilatation.
Therapeutic Challenges
Because of the lack of sophisticated data on carotid FMD, the following challenges frequently arise in its management and must be considered during the treatment planning stage.
3. When symptomatic carotid FMD is treated, it can be a challenge to decide how to manage a contralateral severe but asymptomatic lesion. Although asymptomatic lesions seem to have a generally benign course, such may not be the case in a patient who suffered a stroke on the contralateral side from the same pathology.
5. The presence of an intracranial aneurysm may alter the treatment sequence or the surgical approach.
Clinical Considerations
Because of the relative safety and effectiveness of mechanical intervention (see “Mechanical Repair”), dilatation of the artery is appropriate for lesions causing focal ischemic events (hemispheric or ocular) or episodes of cerebral hypoperfusion. A lesion causing a focal cerebral ischemic event should be considered for treatment because it remains a significant threat. Hypoperfusion is rare but can occur in the setting of critical bilateral carotid FMD, or even unilateral disease when there is a significant defect in the circle of Willis. Percutaneous transluminal angioplasty (PTA) has been successful in the treatment of renal artery FMD, and carotid angioplasty with stenting has a growing role in the management of carotid disease; however, the results and durability of balloon angioplasty for carotid FMD, with or without stent placement, are not known. Symptomatic patients with high surgical risk should be considered for percutaneous balloon angioplasty, especially if the anatomy allows the use of cerebral protection devices.
Anatomic Considerations
Cerebral Protection.
The lesion requiring treatment in carotid FMD is usually a series of webs with intervening pockets, in between which platelet thrombi and cellular debris have accumulated. Disruption of the webs, whether by balloon dilatation or rigid dilatation, produces potentially embolic debris. Some type of cerebral protection is warranted. Carotid FMD tends to involve the carotid artery for several centimeters distal to the bifurcation and may affect the length of the artery to the base of the skull, thus precluding a “landing zone” for an embolic filter to be placed during the procedure. Most distal filters require a few centimeters of straight, healthy artery proximal to the petrous portion of the carotid artery to be functional and safely placed. Cerebral protection using flow reversal devices and proximal occlusion of the common and external carotid arteries may be the best method of protection during percutaneous intervention because the safety and efficacy of these devices has been demonstrated in patients undergoing carotid angioplasty and stenting for atherosclerotic occlusive disease.39
Stents
It is not clear whether carotid balloon angioplasty should be accompanied by stent placement in FMD. Stent placement is not usually required after PTA in renal artery FMD because the results are excellent without stents and because the patients are usually otherwise young and healthy with good life expectancies. However, stents are routinely placed when atherosclerotic stenosis of the carotid bifurcation is treated percutaneously, and there is some likelihood that stent placement after PTA helps stabilize flaps and disrupted dysplastic tissue. Experience with PTA for carotid FMD is limited, and there are even fewer cases of carotid stent placement for FMD. In light of this paucity of cases, more time will be required to determine the role of PTA with or without stenting in the treatment of patients with carotid FMD.
Treatment
Medical Management
Patients with asymptomatic carotid stenosis secondary to FMD should be started on a regimen of antiplatelet agents. Diagnostic evaluation should be undertaken to rule out other arterial pathology. If associated conditions are identified, either in the carotid circulation (e.g., carotid aneurysm) or in other vascular beds (e.g., renal artery stenosis), they should be treated as needed.
Carotid FMD should be monitored at intervals of 6 months with a noninvasive study such as DUS, CTA, or MRA. If DUS follow-up is used, the internal carotid artery must be interrogated as distally as possible. If symptoms develop or there is a significant change in the pathologic lesion (e.g., dissection), mechanical repair of the artery should be considered. There is no established role for anticoagulation or for anti-inflammatory medications such as steroids in carotid FMD. Chiropractic manipulation of the neck should be avoided as should sports that are likely to produce whiplash-type neck injuries.
Mechanical Repair
Methods of mechanical intervention for the treatment of carotid FMD include open surgical graduated rigid dilatation, open access for transluminal balloon dilatation with proximal clamping and back-bleeding to avoid embolization, and PTA (with or without stent placement or cerebral protection devices). Although open surgical treatment has been successfully used in the past, percutaneous balloon therapy with flow reversal represents the optimal current approach, as discussed later. Surgical therapy or endovascular therapy with stents or coils may also be needed for patients with concomitant aneurysms or with aneurysmal complications.
Open Surgical Dilatation.
The usual fibrodysplastic lesion encountered in the internal carotid artery responds to mechanical dilatation. Over the past several decades such treatment has been performed with relative success and safety by means of rigid dilators of progressively enlarging size passed antegrade into the internal carotid artery with arterial control.1,7,26,30,31 This approach permits gentle disruption of the obstructive webs while allowing associated debris to be flushed out of the artery and has been shown over the years to have reasonable results.
The main disadvantage of this approach is that it is performed without imaging of the luminal surface. The length of the arterial segment to undergo treatment must be estimated. Kinks and coils must be managed by “feel” without direct guidance. There is no simple method of assessing the results of treatment in real time, the way interval arteriography can guide a procedure during percutaneous treatment. The other disadvantage is the need for a neck incision and arterial exposure.
Exposure for the open approach is similar to that for carotid endarterectomy, except that higher internal carotid artery exposure is usually required to ensure that dilatation is carried out under direct vision and that the extracranial carotid artery can be safely straightened during passage of the dilator (see Chapter 100). The posterior belly of the digastric muscle may be divided, but subluxation of the mandible is rarely required. If the distal internal carotid artery is not accessible, balloon angioplasty should be considered (see “Open-Access Balloon Dilatation”). The normal arterial segment above the highest point of involvement is apparent with direct inspection, and the internal carotid artery is encircled at this point.
The surgeon should take care to not manipulate the intervening segment of the internal carotid artery as it is gently exposed throughout its length. Determination of stump pressure or electroencephalographic monitoring is not ordinarily needed for this brief procedure but may be indicated if a more extensive procedure is planned (e.g., bifurcation endarterectomy, correction of redundancy, interposition grafting). Except in such unusual circumstances, a shunt is unnecessary.
Heparin is administered (75 to 100 U/kg) before flow is interrupted. Dextran 40, 25 mL/hr beginning at the time of surgery and continued during the immediate postoperative period, may help prevent early deposition of thrombotic material on the inner surfaces of the arteriotomy and the fractured septa. The common carotid artery is cross-clamped. Traction on a polymeric silicone (Silastic) “sling” placed around the internal carotid artery just above the bifurcation is performed to straighten the artery. A short arteriotomy is made in the internal carotid artery at the base of the bulb. Graduated metal dilators are then gently passed up the straightened internal carotid artery, beginning with a 1.5-mm-diameter probe and progressing up to a 3.5-mm- or, occasionally, a 4.0-mm-diameter probe (Fig. 102-4). A series of “giving” sensations is usually felt as each septal stenosis is gently fractured for the first time, but such sensations are not felt thereafter.
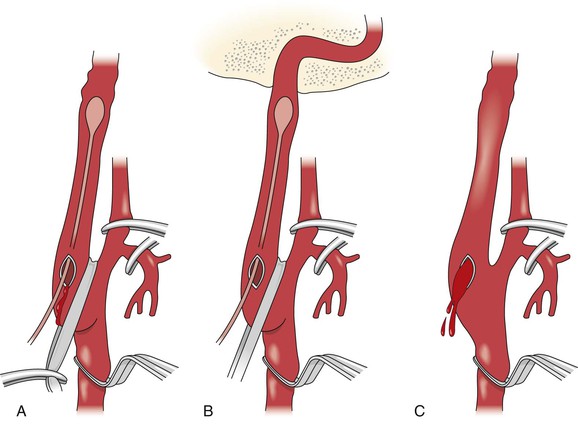
Figure 102-4 Drawings showing the main features of the open surgical technique. A, Straightening of the carotid artery with downward traction on a polymeric silicone (Silastic) sling. B, Gentle, graduated dilatation of the internal carotid artery from 2 to 4 mm. Passage of the dilator to the bony canal at the base of the skull is shown. C, Back-bleeding of the artery to remove dislodged debris. (From Wylie EJ, et al: Nonatherosclerotic diseases of the extracranial carotid arteries. In Manual of vascular surgery, New York, 1986, Springer-Verlag, pp 184-185.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree