Roger J. Hajjar, Joshua M. Hare
Cardiovascular Regeneration and Gene Therapy
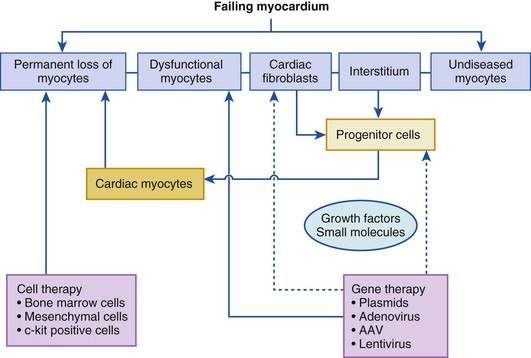
A longstanding quest in cardiovascular therapeutics is to regenerate injured tissue or to correct fundamental molecular defects in signaling pathways that cause organ dysfunction in the setting of heart failure. Regardless of the specific etiologic disorder, the failing myocardium is composed of diseased cardiac myocytes, permanently lost cardiac myocytes that are replaced by fibrous tissue, and normal cardiac myocytes. As shown in Figure 30-1, the goal of cell therapy is to replace the permanently lost cardiac myocytes within the myocardium, whereas the goal of gene therapy is to improve the function of the failing cardiac myocytes by modulating the expression of specific genes. Significant overlap exists between gene and cell therapies. Genetically modified cells have been shown to have better survival and differentiation capacity once transplanted into the myocardium. In addition, direct differentiation of fibroblasts into myocytes using gene therapy has been shown experimentally, and expression of secreted factors that induce homing of progenitor cells can be achieved by myocardial gene therapy. After decades of research, the feasibility of both of these strategies is supported by clinical and translational data.
At present, both cell and gene therapies are experimental approaches to treating heart disease. Although few late-stage clinical trials have been completed, much progress has been made in terms of delivery strategies and patient selection profiles that will shape the ultimate clinical application of cell and gene therapy in cardiovascular medicine.
Cell Therapy
Principles of Cell- and Gene-Based Cardiac Regenerative Therapies
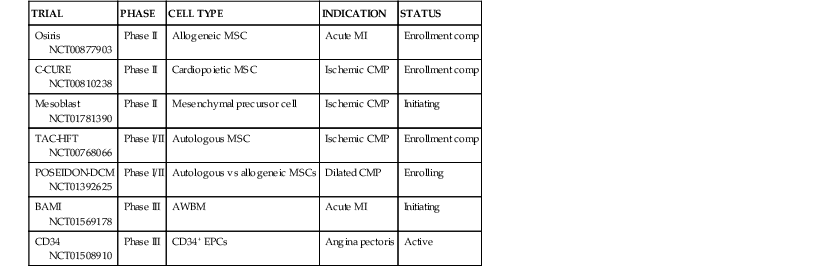
On first principles, the goal of cardiac cell-based therapy is to repopulate areas of damaged myocardium with cells capable of engraftment and trilineage differentiation into cardiac myocytes, vascular smooth muscle, and endothelium, whereas the goal of gene therapy is to alter gene expression within the myocardium to enhance cardiac function. Data from basic, preclinical, and clinical studies have established the principle that successful cell-based tissue repair results from an integrated orchestration of cellular and molecular events. Gene therapy approaches have targeted critical pathways that are altered in cardiovascular diseases. The initial clinical trials that tested cell-based therapy for heart disease used autologous whole bone marrow (AWBM), skeletal myoblasts, and mesenchymal stem cells (MSCs). A second wave of therapeutic approaches, many of which are still being investigated in ongoing studies, use cardiac stem cells (CSCs), mesenchymal precursor cells (MPCs), and cell combinations (Table 30-1). Clinical trials in gene therapy for cardiovascular diseases have been fewer, but with the development of novel vectors and the identification of novel targets, gene-based therapies are being contemplated for cardiovascular diseases. Extensive development work has laid the groundwork for catheter-based transendocardial and transmyocardial stem cell injection. Together, the advancements made in the past decade make it possible to envision the widespread availability of cell-based therapy for a panoply of cardiac disorders currently considered chronic and incurable.
The concept of treating a wide range of human heart diseases with a regenerative strategy has been advanced by both basic biologic and translational research over the past decade.1,2 The idea that cell repopulation, if achievable, could be an effective therapeutic strategy has as its underpinning the paradigm that the human heart, unlike that of amphibians, fish, and possibly lower mammals,3 is terminally differentiated and incapable of regeneration in postnatal life (i.e., the human heart is a postmitotic organ). Of interest, a series of recent studies have shown that the human heart does possess the capacity for turnover of myocytes, although the rate of this process varies among the different studies.3–5 As described next, this endogenous regenerative capacity plays a critical role in cell-based tissue repair.
The approaches to finding an effective cell-based therapy have evolved considerably over the past decade. First, major attention has shifted from embryonic/pluripotent stem cells toward sources of adult cells that could have the capacity for cardiac tissue repair.6,7 Second, it is now clear that many other facets of cell-based therapy have the potential to contribute to the success of the approach, and these include antifibrotic effects, neovascularization, and the stimulation of endogenous CSCs.8–12 Finally, attention has been paid to advancing the practical aspects of cell delivery through the development of effective cell delivery systems and advances in cell production; effective delivery is likely to play a pivotal role in successful translation of this new strategy.6,12 Gene and vector delivery systems also have evolved considerably since the first gene therapy trial for the treatment of monogenic diseases. The molecular targets for therapeutic intervention also have increased significantly over the past 2 decades for specific aspects or elements of cardiac pathophysiology. The potential of novel gene transfer technology and the demands imposed by the cardiac pathophysiology of interest are discussed in this chapter.
Cell Types Used—Past, Present, and Future Strategies
First-Generation Cell Therapeutics
Autologous Whole Bone Marrow Cells
Immediately after publication of the seminal report in 2001 by Orlic and co-workers that murine bone marrow c-kit–positive cells could repair the infarcted murine heart,13 a wave of clinical trials ensued to test the hypothesis that AWBM could improve the structure and function of the post–myocardial infarction (MI) heart.9 The totality of evidence from these trials suggests that intracoronary AWBM administered to patients after MI increased left ventricular ejection fraction (LVEF) by 2% to 3%, in an effect that increased with greater degrees of LVEF decline after infarction.14,15 The relatively small impact on ejection fraction, coupled with the concern over whether bone marrow–derived cells can effectively differentiate into cardiac myocytes, promoted a quest for additional cell types. Despite the fact that data for myocyte differentiation are lacking, analyses from clinical trials suggest that intracoronary AWBM can have clinical benefits, reducing heart failure hospitalization and reinfarction rates.16 As a result, the merit of AWBM as a clinical therapy will be subjected to a pivotal phase III study, the Effect of Intracoronary Reinfusion of Bone Marrow–Derived Mononuclear Cells (BM-MNC) on All-Cause Mortality in Acute Myocardial Infarction (BAMI) trial (NCT01569178). For this 3000-patient, randomized phase III trial, conducted in Europe, mortality is the primary outcome variable (see Table 30-1).
Mesenchymal Stem Cells
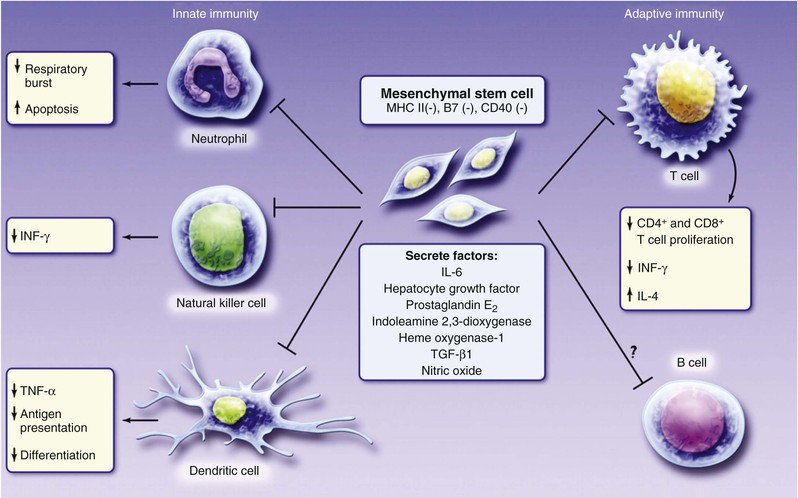
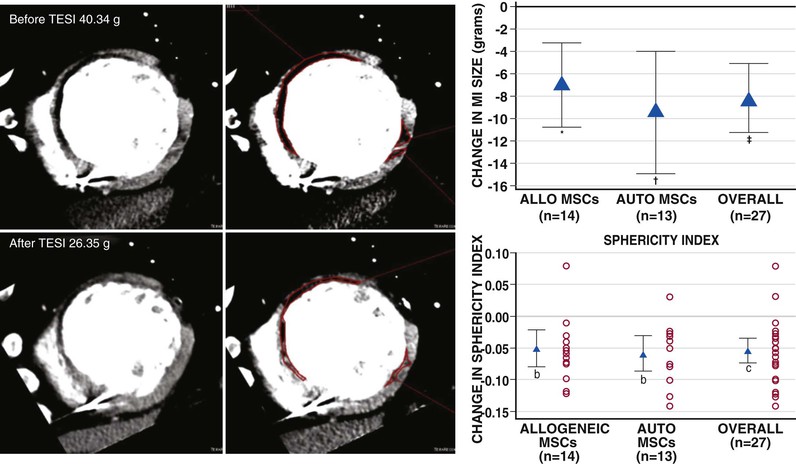
Another notable strategy undergoing early-stage clinical testing is the use of MSCs. These adult stem cells are prototypically found in the bone marrow, from which they can be cultured. MSCs are niche-regulating cells, widely distributed in adipose tissue, umbilical cord blood, endometrium, and other sources,1 that have the capacity for multipotent differentiation and neovascularization. MSCs have been tested in phase I trials for treatment of acute MI,17 proof-of-concept studies for treatment of ischemic heart failure,6 and phase I and phase II clinical trials for treatment of heart failure due to ischemic and nonischemic cardiomyopathy and acute MI. MSCs are particularly attractive as a cell therapeutic, because they are immunoprivileged (Fig. 30-2) and therefore have potential as an “off the shelf” therapeutic.9 In a study conducted in patients with chronic ischemic cardiomyopathy that compared allogeneic and autologous bone marrow–derived MSCs, the immunologic profile of the MSCs was acceptable, and both types of cells had similar efficacy (Fig. 30-3). In two studies, MSCs (both allogeneic and autologous) reduced MI size by ~33%, improved indices of myocardial remodeling, and improved patient quality of life and functional capacity.18,19
In addition to bone marrow–derived cells, MSCs derived from alternative tissue sources, notably fat, also are currently under evaluation for use as cardiac therapeutics.20 The use of adipose-derived MSCs is advantaged by the abundance of these stem cells in adipose tissue, allowing isolation of adequate numbers of cells without requiring expansion.
Skeletal Myoblasts
Skeletal myoblasts represent a third cell type that has undergone clinical testing. After completion of several early-stage trials suggesting benefit, a phase II investigation involving the random assignment of 97 patients to specific treatments failed to show significant clinical benefit. Thus the approach of using autologous skeletal myoblasts has an uncertain future.
Endothelial Precursor Cells (CD34+ Cells)
Another application of cell-based therapy has entailed a neo-angiogenesis strategy using CD34+ endothelial precursor cells in patients with chronic angina pectoris and acute MI.21,22 This strategy is based on the idea that these cells can generate microvasculature in poorly perfused tissue sections. Abundant support for this hypothesis has accrued, and a recent phase II trial provides insights into the possibility of clinical efficacy.23 The success of the phase II trial of CD34+ cells has paved the way for an ongoing phase III study that will test the clinical impact of this form of cell therapy (see Table 30-1).
Second-Generation Cell Therapeutics
The field of cell-based therapy is rapidly advancing with regard to the quest for more effective cell products, and numerous strategies are now entering proof-of-concept early-stage clinical investigations. An approach to advancing MSC strategies involves identifying an MSC precursor cell in a tissue source, usually the bone marrow. In addition to culturing MSCs, MPCs can be obtained through epitope-based cell enrichment techniques, including the use of Stro-1, Stro-3,24 and potentially CD271. Other second-generation MSC-related cells include multipotent adult precursor cells (MAPCs)25,26 and cardiopoietic MSCs.27 Whereas MAPCs are MSC-like cells that are more primitive and therefore are theorized to have a greater differentiation capacity, cardiopoietic MSCs are MSCs that are cultured with a cocktail of cardiopoietic cytokines shown to enhance the ability of the MSC to differentiate in vitro and in vivo. With regard to clinical testing, Stro-3 MPCs and MAPCs are each in phase II testing for a variety of indications, including ischemic heart failure and/or acute MI (see Table 30-1). In the recently completed C-CURE trial of cardiopoietic MSCs, intramyocardial administration of these autologous cells increased ejection fraction, reduced end-systolic volume, and improved both the 6-minute walk distance and a clinical composite score.27
Cardiac Stem Cells
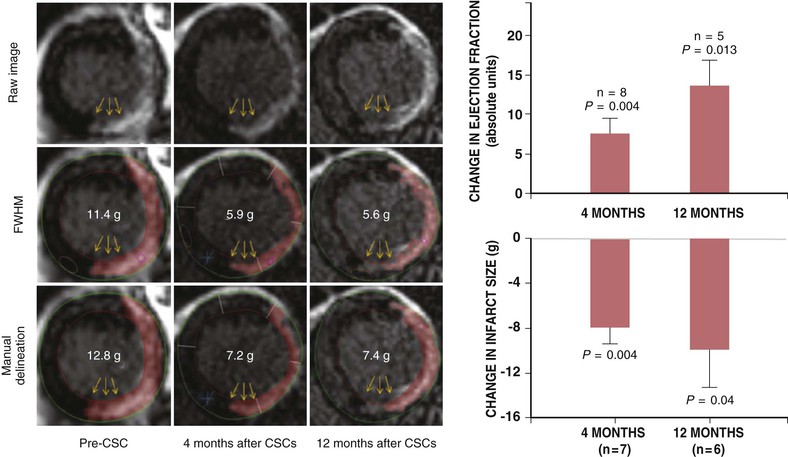
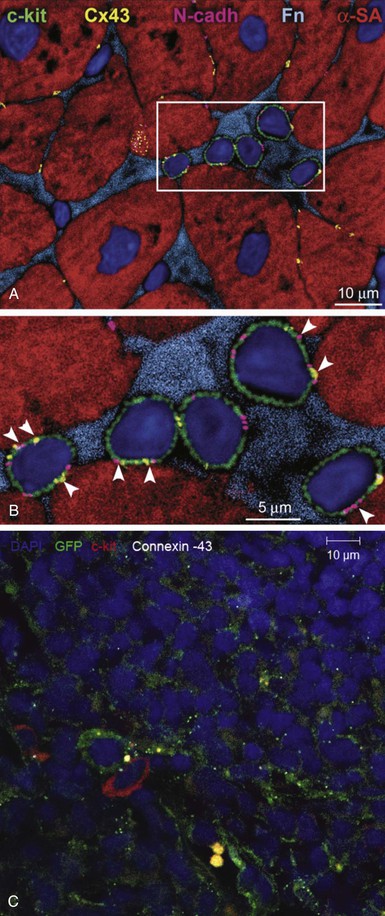
One of the most exciting and transformative discoveries of the past decade is that of the adult CSC.28 A compartment of stem cells capable of trilineage differentiation has been described in the mammalian heart that bears the c-kit receptor.28 These cells exist in niches, akin to niches found in other organ systems (Fig. e30-1![]() ). Other CSCs are defined by expression of the Isl-1 transcription factor (found in the fetal heart—probably a second heart field precursor), the Sca-1 receptor (found in lower mammals without a human analogue), cells expressing the WT1 transcription factor, which are described to be in the subepicardium.30,31 Of these cells, c-Kit positive CSCs are the best characterized and have entered early-stage clinical testing. A pure preparation of CSCs, amplified from autologous atrial appendage samples, was tested for clinical safety in the Cardiac Stem Cell Infusion in Patients with Ischemic CardiOmyopathy (SCIPIO) trial.32,33 Intracoronary infusion of autologous cardiac c-Kit cells resulted in an increase in ejection fraction and a decrease in infarct size, both of which were sustained over a 2-year period (Fig. 30-4).7 Advances in the biology of CSCs have led to further characterization of c-kit–positive CSCs. Recently it was identified that c-kit–positive CSCs bearing the insulin growth factor (IGF)-1 receptor are more cardiopoietic.34 In addition, it is clear that CSCs comprise subpopulations of vasculogenic and cardiopoietic cells.35
). Other CSCs are defined by expression of the Isl-1 transcription factor (found in the fetal heart—probably a second heart field precursor), the Sca-1 receptor (found in lower mammals without a human analogue), cells expressing the WT1 transcription factor, which are described to be in the subepicardium.30,31 Of these cells, c-Kit positive CSCs are the best characterized and have entered early-stage clinical testing. A pure preparation of CSCs, amplified from autologous atrial appendage samples, was tested for clinical safety in the Cardiac Stem Cell Infusion in Patients with Ischemic CardiOmyopathy (SCIPIO) trial.32,33 Intracoronary infusion of autologous cardiac c-Kit cells resulted in an increase in ejection fraction and a decrease in infarct size, both of which were sustained over a 2-year period (Fig. 30-4).7 Advances in the biology of CSCs have led to further characterization of c-kit–positive CSCs. Recently it was identified that c-kit–positive CSCs bearing the insulin growth factor (IGF)-1 receptor are more cardiopoietic.34 In addition, it is clear that CSCs comprise subpopulations of vasculogenic and cardiopoietic cells.35
C-Kit positive CSCs may be prepared from either surgical specimens of the atrial appendage33 or endomyocardial biopsy samples of right ventricular septum.7 Collagenase-digested tissue yields c-Kit cells purified by antigenic panning, which are then expanded in culture, yielding therapeutic quantities of cells. After culture expansion, the degree of c-Kit expression may fall. As discussed further on, recognition of the presence of CSCs within the heart has offered a major insight into the mechanism of action of MSCs to stimulate myogenesis.2
From a clinical testing perspective, another approach in cell-based therapy involves culture expansion of cell collections that have been called cardiospheres. These cell collections, which may be obtained from heart biopsy material, have been shown in the CADUCEUS trial (in patients with ischemic left ventricular dysfunction after a recent MI) to reduce infarct size. Further trials are anticipated for this approach.