61 Cardiovascular Manifestations of Endocrine Diseases
Pituitary Gland Disorders
The seven peptide hormones secreted by the anterior pituitary gland and two secreted by the posterior pituitary gland all affect the cardiovascular system. Most indirectly cause changes in salt or water metabolism or affect vascular tone. The anterior pituitary hormones and their direct and indirect effects on cardiovascular function are listed in Table 61-1. Three disorders can result in major changes in cardiovascular function: hypopituitarism, acromegaly, and disorders of antidiuretic hormone (ADH) secretion.
Table 61-1 Pituitary Hormones and Their Actions on the Cardiovascular System
| Hormone | Direct | Indirect |
|---|---|---|
| ACTH | ||
| TSH | Stimulates thyroxine and triiodothyronine synthesis | Thyroxine stimulates HR, pulse pressure, and LV contractility. |
| LH | Stimulates estrogen and testosterone synthesis | Estrogen acts as a vasodilator. |
| ADH | Stimulates water retention, increases plasma volume; acts through a central mechanism to increase vasoconstriction | |
| GH | Stimulates vasomotor force and LV function | Through IGF-1, it stimulates HR. |
ACTH, adrenocorticotrophic hormone; ADH, antidiuretic hormone; GH, growth hormone; HR, heart rate; IGF-1, insulin-like growth factor I; K+, potassium; LH, luteinizing hormone; LV, left ventricular; Na+, sodium; TSH, thyroid-stimulating hormone.
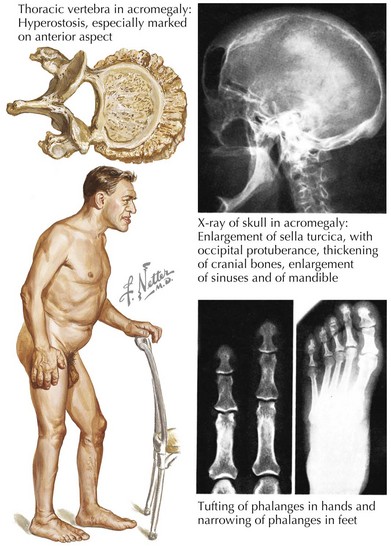
Acromegaly
Sustained hypersecretion of GH by a pituitary tumor can lead to overgrowth of several tissues and to considerable cardiovascular changes (Fig. 61-1). Cardiovascular function is an important determinant of morbidity and mortality in untreated acromegaly. The most common comorbid cardiovascular condition accompanying acromegaly is hypertension, present in 50% of inadequately treated patients. Hypertension in acromegaly is usually mild but can be difficult to manage conventionally. Left ventricular (LV) mass can be significantly increased compared with that of normotensive patients. Curing the acromegalic condition is the most effective way to lower blood pressure (BP). A concentric ventricular hypertrophic cardiomyopathy unassociated with hypertension but associated with long-standing acromegaly develops in some patients and can result in both diastolic and systolic dysfunction. Cardiomegaly can be disproportionate to the changes in size that occur in other organs in severe acromegaly. The severity of cardiomyopathy correlates with the duration of exposure to high levels of GH. Diastolic dysfunction and hypertrophy develop first and are common in untreated patients. These changes are reversible with adequate treatment of GH excess. If left untreated, there is progression to systolic dysfunction, and heart failure and severe ventricular arrhythmias can occur. Histologic evaluation of the myocardium in patients with long-standing acromegaly shows interstitial fibrosis, lymphocytic infiltration, and sometimes necrosis.
Thyroid Disorders
Hyperthyroidism
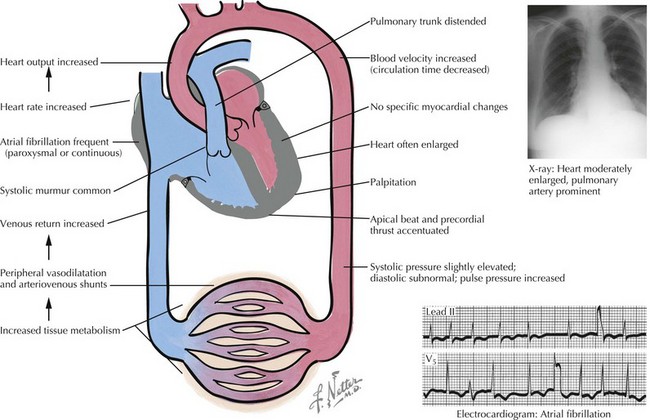
The symptoms of cardiac dysfunction that occur most commonly in thyrotoxicosis include fatigue, palpitations, dyspnea, heat intolerance, increased sweating, and weight loss. Tachycardia and palpitations occur in 80% to 90% of untreated patients (Fig. 61-2). Elderly patients in whom Graves’ disease develops may also experience heart failure. In this circumstance, the failing heart cannot meet metabolic requirements that are raised by increased thyroid hormone, resulting in overt congestive heart failure (CHF). Similarly, angina pectoris may be an important symptom in elderly patients with hyperthyroidism. Myocardial oxygen consumption can increase by as much as 70% in untreated hyperthyroidism. In the presence of fixed coronary lesions, blood flow may be inadequate to supply the increased metabolic need. In younger patients, thyrotoxicosis is associated with increased inotropic and chronotropic effects on the heart. Palpitations and occasionally atrial arrhythmias are the initial symptoms. Atrial fibrillation occurs in 33% to 47% of patients who are older than 60 years. Vascular resistance is decreased by peripheral vasodilation; the net effect is a marked increase in CO, which results in increased oxygen consumption. Peripheral edema is the most common symptom of overt heart failure in Graves’ disease, although dyspnea on exertion can also be prominent.