Martha Gulati, C. Noel Bairey Merz
Cardiovascular Disease in Women
Cardiovascular disease (CVD) remains the leading cause of death in women. More women than men have died annually from CVD since 1984 in the United States, with coronary heart disease (CHD) accounting for 401,495 deaths in women in 2009.1 A total of 42,900,000 women are living with some form of CVD, including hypertension, and the lifetime risk for development of CVD in a 40-year-old woman is estimated to be 1 in 2—with a 1 in 3 risk for the development of CHD, 1 in 5 risk for the development of heart failure (HF), and 1 in 5 risk of having a stroke in their lifetime.1 Since 2001, mortality from heart disease has continuously declined in women,1 but in younger women (<45 years), mortality from heart disease has actually increased.
There are both sex (biologic) and gender (sociocultural) differences in CVD, and differences in outcomes between women and men are due to a number of variables, including specific CVD risk factors in women, differences in treatment and management strategies in women for both primary and secondary prevention of CVD, and pathophysiologic differences in CVD.
Despite the fact that more women than men have been dying of CVD in the United States, it was not until 1991 that the National Institutes of Health (NIH) established a policy that all NIH-funded trials must include both women and men in studies of conditions that affect both sexes. Most of the studies on women and CVD commenced following this mandate by Dr. Bernadine Healy, director of the NIH at that time. Although awareness that CVD is the leading cause of death in women has increased from 1997 to 2012 (30% versus 56%, P < 0.001), it has remained suboptimal since 2006, particularly in racial and ethnic minorities. Physician awareness of women’s risk for CVD is also not at goal. In a study from 2007, only 71% of surveyed internists and obstetric gynecologists responded correctly to all 13 questions that assessed knowledge about cardiac risk factors.
Sex, Gender, and Genetic Differences in Cardiovascular Disease
The Institute of Medicine has defined sex as “the classification of living things, generally as male or female according to their reproductive organs and functions assigned by the chromosomal complement.”2 Sex differences result from true biologic differences in the structure and function of the cardiovascular systems of men and women, in contrast to gender differences, which stem from a person’s self-representation resulting from the psychosocial roles and behavior imposed by society.
Genetic markers predictive of CVD have not been defined in women to date. The Women’s Health Genome Study monitored 19,313 white women prospectively for a median of 12.3 years to assess whether a genetic risk score could improve the predictive risk assessment of women beyond the traditional risk factors.3 They were unable to show an improvement in CVD risk prediction in women with their comprehensive literature-based genetic risk score. This was true whether the component genetic effects were extended to include polymorphisms acting on intermediate phenotypes or were restricted only to those directly associated with CVD outcomes.
Cardiovascular Risk Factors in Women (see also Chapter 42)
Established Risk Factors for Cardiovascular Disease
Age
Age powerfully predicts CVD and, specifically, CHD. The prevalence of CVD increases with age in both men and women, but CHD events in women occur on average approximately 10 years after those in men. CHD increases in women older than 60 years, with one in three women older than 65 years having evidence of CHD, in contrast to one in eight women 45 to 64 years of age. The National Cholesterol Education Program Adult Treatment Panel (NCEP ATP III) considers age 55 years or older to be a risk factor for women, as compared with 45 years for men. Nonetheless, the highest sex difference in CHD mortality is observed in relatively young middle-aged women, in whom mortality from acute myocardial infarction (AMI) is twice that of age-matched men; in contrast, no sex difference is seen in elderly women and men.
Family History
A history of CHD in a first-degree relative increases risk. The NCEP ATP III and the American Heart Association (AHA) guidelines for the prevention of CVD in women define a family history of premature CHD as a first-degree relative with CHD before 65 years of age for women and before 55 years for men. Premature CHD in first-degree female relatives is a relatively more potent family history risk factor than is premature CHD in male relatives. In addition, women classified as being at low risk for CHD (using the Framingham Risk Score) but having a sister with premature CHD are more likely to have evidence of subclinical CHD by coronary artery calcium based on a study of 102 asymptomatic women. The 2010 American College of Cardiology Foundation (ACCF)/AHA guideline for assessment of cardiovascular risk in asymptomatic adults recommends that a family history of CVD be obtained for assessment of cardiovascular risk in all asymptomatic adults.
Hypertension
The prevalence of hypertension overall is higher in women than in men but varies by age. Based on data from the National Health and Nutrition Examination Survey (NHANES), before the age of 45, more men than women have hypertension. From 45 to 64 years of age, men and women have a similar prevalence of hypertension, but at 65 years and older, women have a higher prevalence of hypertension than men do. In women taking oral contraceptives, hypertension is two to three times more common than in women not taking them, and use raises blood pressure 7 to 8 mm Hg on average although this is typically well-tolerated and not associated with future development of CVD.4
The NHANES survey from 1999 to 2004 demonstrated that hypertensive women were more likely to be treated than men but were less likely to achieve blood pressure control. In addition to a higher prevalence of hypertension in older women, blood pressure control is also poorer in this population. In the Women’s Health Initiative Observational Study, only 29% of hypertensive women 70 to 79 years of age had blood pressure lower than 140/90 mm Hg as compared with 41% and 37% of those 50 to 59 years of age and 60 to 69 years of age, respectively.
Hypertension is associated with increased risk for the development of congestive HF, but this risk appears to be greater in women. From the Framingham Heart Study and Framingham Offspring Study, risk for the development of HF in those with hypertension versus normotensive subjects was about twofold in men and threefold in women.
Women with strokes are more likely than men to have a history of hypertension.5 This is especially important because the lifetime risk for stroke is greater in women than in men because of their greater life expectancy and because stroke rates increase substantially with age.
Diabetes
Diabetes is a relatively greater risk factor for CHD in women than in men; it increases a woman’s risk for CHD by threefold to sevenfold, with only a twofold to threefold increase in diabetic men.6 In addition, risk for fatal CHD in diabetic women is 3.5 times higher than in nondiabetic women, which is higher than the risk in diabetic men (the 2.0 times higher risk in diabetic to nondiabetic men).6
The American Diabetes Association (ADA) suggests consideration of diabetes screening for women and men older than 45 years and then repeating every 3 years if the results are normal. For women with a history of gestational diabetes, screening for diabetes should occur 6 to 12 weeks postpartum and then every 1 to 2 years thereafter.7
Dyslipidemia
Dyslipidemia is common in women; more than half of U.S. women have total cholesterol greater than 200 mg/dL, and 36% have low-density lipoprotein cholesterol (LDL-C) greater than 130 mg/dL. Notably in women, adverse changes in the lipid profile accompany menopause and include increased levels of total cholesterol, LDL-C, and triglycerides and decreased levels of high-density lipoprotein cholesterol (HDL-C); how much worsening is due to aging versus menopause is unclear.8
The ATP IV guidelines name LDL-C as the primary target of statin lipid-lowering therapy to reduce CVD. Furthermore, the 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults does not recommend measurement of lipoprotein profiles by nuclear magnetic resonance spectroscopy, apolipoproteins, particle size, and density for assessment of asymptomatic adults. These guidelines recommend measuring a fasting lipid panel and treating patients with established CVD, diabetes, or LDL-cholesterol >190 mg/dL with moderate- to high-dose statin therapy. For patients not in these three groups, the guidelines recommend calculation of atherosclerotic cardiovascular disease (ASCVD) risk.8a
HDL-C predicts CVD in both men and women, perhaps more so in women. In the Framingham study, men in the lowest quartile for HDL-C (HDL <36 mg/dL) had a 70% greater risk for myocardial infarction (MI) than did those in the highest HDL-C quartile (HDL >53 mg/dL). However, this risk was even stronger for women with low HDL-C. Women in the lowest HDL-C quartile (HDL <46 mg/dL) had a six to seven times higher rate of coronary events than did those in the highest HDL-C quartile (HDL >67 mg/dL), even after adjustment for other risk factors. HDL-C levels in women average approximately 10 mg/dL higher than in men throughout their lives. Guidelines reflect this difference and specify a desired HDL-C level of 50 mg/dL in women as opposed to 40 mg/dL in men.9
Smoking
In 2009, 23.1% of men and 18.3% of women reported tobacco use, thus placing them at increased risk for CVD. Smoking may be more detrimental in women than in men. Female smokers die 14.5 years earlier than female nonsmokers, and male smokers die 13.2 years earlier than male nonsmokers. Cessation of smoking substantially reduces risk; risk for mortality in former smokers decreases almost to that of never smokers 10 to 14 years after cessation.
The use of oral contraceptives together with smoking imparts an even greater risk for MI than does smoking alone, which is thought to be related to prothrombotic effects. Smoking 25 or more cigarettes per day increases women’s risk by 12- to 32-fold. Third-generation hormonal contraceptives appear to pose lower risk than do the previous and fourth generations.
Physical Activity/Physical Fitness
Physical inactivity is a common risk factor for CHD, but sedentary behavior is more common in women than in men (33.2% versus 29.9%), and inactivity increases with age,1 although sex bias in physical activity measurement instruments that do not assess domestic activities such as cooking, cleaning, and child care may account for the differences observed. Only 17.1% of women met these guidelines, as opposed to 24.9% of men, in the 2011 National Health Interview Survey.1 Between NHANES 1988-1994 and NHANES 2001-2006, the proportion of women who engaged in physical activity 12 or more times per month fell from 49% to 43%. Physical inactivity is a risk factor for CVD given its association with higher blood pressure, worse cholesterol, poorer glucose metabolism, and poorer mental health. Inactivity also contributes to obesity. Physical inactivity is an independent risk factor; the Nurses’ Health Study found that women who walked for 30 to 45 minutes three times per week reduced their risk for MI by 50%, independent of age. In a meta-analysis of studies of physical activity in women, a 43% lower risk for new CHD was observed in women in the highest physical activity group than in the least active women.
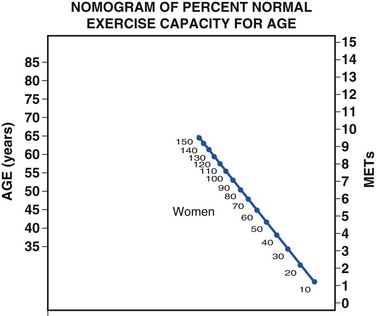
In the St. James Women Take Heart Project, asymptomatic women who were unable to achieve 5 metabolic equivalents (METs) on a Bruce protocol had a threefold increased risk for death when compared with women who achieved greater than 8 METs. The risk for death in asymptomatic and symptomatic women whose exercise capacity was less than 85% of the predicted value for age was at least twice that of women whose exercise capacity was 85% or greater of their age-predicted value.10 Assessment of age-predicted fitness can be estimated by using the validated nomogram in Figure 77-1.
Emerging Risk Factors
Metabolic Syndrome
NHANES data from 2003 to 2006 indicate that 32.6% of women met the criteria for metabolic syndrome. In addition, those with metabolic syndrome have an increased risk for the development of CVD, and this association is strongest in women, with a relative risk for CHD of 2.63 as compared with 1.98 in men.
Obesity
Obesity, defined as a body mass index (BMI) greater than 30 kg/m2, is epidemic in the United States, with the 2009-2010 NHANES estimation of obesity in women being 36%. The rising incidence of diabetes is closely associated with obesity. Data from the Framingham Heart Study demonstrated a doubling of the incidence of diabetes over the past 30 years, most dramatically in the 1990s and primarily in individuals with a BMI higher than 30 kg/m2. In the Nurses’ Health Study, obesity was the most powerful predictor of diabetes. Women with a BMI of 35 kg/m2 or higher had a relative risk for diabetes almost 40-fold greater than that in women with a BMI lower than 23 kg/m2.11 The pattern of obesity appears to be related to CVD: waist circumference above 35 inches, indicative of visceral obesity, is related to elevated risk for CVD, whereas elevated BMI alone is not.12
Obesity is associated with increased mortality from CVD, with the 2004 NHANES data demonstrating a 13% increased risk for cardiovascular death in obese women versus those with a normal BMI. Obesity is also associated with a decrease in life expectancy in women, as demonstrated in the Framingham Heart Study, in which a 40-year-old nonsmoking woman was found to lose 7.1 years of life expectancy as a result of obesity. Despite these data, obesity is not an independent risk factor for CVD, although it is strongly associated with many of the traditional risk factors for CHD. Notably, overweight, defined as a BMI greater than 25, but not obese as defined as greater than 30, is associated with lower mortality and CVD death than in those of normal weight. Previous studies in women in whom both obesity and physical fitness were measured have suggested that obese women who are physically fit are not at elevated risk and, conversely, that lean women who are not physically fit have elevated risk.13
High-Sensitivity C-Reactive Protein
Whether high-sensitivity C-reactive protein (hsCRP) is a causal risk factor for CVD is unclear, but it may improve risk detection in women.14 In the Women’s Health Study, a global risk prediction model that included hsCRP improved prediction of risk for CVD.14 In one study of apparently healthy women, those with metabolic syndrome and hsCRP levels higher than 3.0 mg/L had almost twice the risk for future cardiovascular events than did those with metabolic syndrome and hsCRP lower than 3.0 mg/L. Measurement of hsCRP is not currently recommended for routine risk assessment of women but rather as an option in those in the intermediate risk range based on the Framingham Risk Score.
Autoimmune Disease (see also Chapter 84)
The systemic inflammation associated with autoimmune disease may accelerate atherosclerosis. Rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) have been associated with a significantly increased relative risk for CVD. Women 18 to 44 years of age with SLE are 2.27 times more likely than their age-matched peers without SLE to be hospitalized because of AMI, 3.80 times more likely to be hospitalized because of congestive HF, and 2.05 times more likely to be hospitalized because of a cerebrovascular accident. Women 35 to 44 years of age with SLE in the Framingham Offspring Study were 50 times more likely to have an AMI than were women of the same age without SLE.
Polycystic Ovary Syndrome
Unique to women, polycystic ovarian syndrome (PCOS) is associated with the development of many features of metabolic syndrome, as well as with insulin resistance, although first-degree male relatives also appear to have more insulin resistance. Women with PCOS have an increased prevalence of impaired glucose tolerance, metabolic syndrome, and diabetes when compared with women without PCOS. Even though it is unclear whether PCOS is an independent risk factor, recent data support this.15
Functional Hypothalamic Amenorrhea
Up to 10% of premenopausal women have documented ovarian dysfunction, with a larger proportion having subclinical hormonal dysfunction. Functional hypothalamic amenorrhea (FHA) can cause premenopausal ovarian dysfunction and occurs when gonadotropin-releasing hormone increases, thereby increasing luteinizing hormone in a pulse frequency and causing amenorrhea and hypoestrogenemia. In a large cohort study, women with menstrual irregularities had a 50% increased risk for nonfatal and fatal CHD when compared with women who had regular menstrual cycling. Additional data from women undergoing coronary angiography indicate that FHA is associated with premature coronary atherosclerosis16 and that use of oral contraceptive therapy may confer protection.17 These findings suggest that amenorrhea and cycling irregularity may be a risk factor for CVD in women.
Preeclampsia and Pregnancy-Associated Hypertension
Women with preeclampsia have a 3.6- to 6.1-fold greater risk for the development of hypertension and a 3.1- to 3.7-fold higher risk for the development of diabetes.18 Preeclampsia is also a risk factor for future ischemic stroke. Women with a history of preeclampsia have approximately double the risk for subsequent ischemic heart disease (IHD), stroke, and venous thromboembolic events over the 5 to 10 years following the pregnancy.19
Gestational Diabetes
A history of gestational diabetes doubles the risk for diabetes in the first 4 postpartum months and remains a life-long risk factor for diabetes and CVD.7 Fasting glucose levels of 121 mg/dL or higher during pregnancy increase the risk for diabetes in the early puerperium by 21-fold.
Breast Cancer Therapy
Recent advances in breast cancer treatment have led to improved survival but elevated risk for CVD (see also Chapter 69).20 Breast cancer therapies are associated with varying degrees of direct cardiovascular injury, in conjunction with lifestyle changes that also reduce cardiovascular reserve.20 Whether breast cancer overall or specific therapies for breast cancer per se will emerge as risk factors for CVD is uncertain, but this issue has become increasingly important in the management of women surviving breast cancer. Further work is needed to glean the relative and absolute risk, as well as optimal assessment and treatment strategies, for cardiovascular physicians who will increasingly be called on to evaluate and treat these women.
Reproductive Hormone Therapy
The American College of Obstetricians and Gynecologists (ACOG) and the World Health Organization (WHO) have published guidelines on medical eligibility for contraceptive use. For most women who are healthy and free of CVD and cardiovascular risk factors, the use of combination estrogen-progestin oral contraceptives is associated with low relative and absolute risks for CVD. Smokers and women with uncontrolled hypertension, IHD, and obesity have an unacceptable level of risk associated with oral contraceptives.4
CVD in the menopause occurs in association with an increased burden of established CVD risk factors.21 Even though postmenopausal hormone therapy was hypothesized to reduce the incidence of CVD, multiple randomized trials did not find hormone therapy or selective estrogen receptor modulators (SERMs) to primarily or secondarily prevent CVD primarily or secondarily. The “Effectiveness-Based Guidelines for the Prevention of Cardiovascular Disease in Women—2011 Update: A Guideline from the American Heart Association” states that hormone replacement therapy and SERMs should not be used for the primary or secondary prevention of CVD and are a class III, level of evidence A intervention.
Assessment of Risk for Cardiovascular Disease
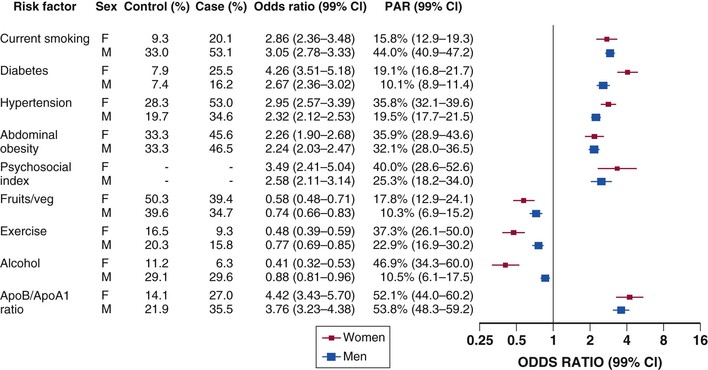
INTERHEART, a large case-control study, examined the association of multiple risk factors with risk for MI and compared the relative risk of their association by sex.22 This study found that nine factors accounted for 94% of the population-attributable risk for AMI in women and 90% of the risk in men. These risk factors included the apo B/apo A-I ratio, cigarette smoking, hypertension, diabetes, abdominal obesity, psychosocial factors (index score based on depression, stress at home or work, financial stress, and life events and a control score), fruit and vegetable intake, exercise, and alcohol intake. Diabetes and psychosocial factors had a greater association with risk for AMI in women, and exercise, fruit and vegetable consumption, and modest alcohol consumption were associated with greater prevention of AMI in women than in men (Fig. 77-2).22
Guidelines recommend the use of a global risk score (such as the Framingham Risk Score) with multiple traditional cardiovascular risk factors for all asymptomatic adults without a clinical history of CHD as a class I indication. Other risk scores also exist (see Chapter 42). The Reynolds Risk Score calculates risk in women and men; it includes hsCRP and family history as risk factors and considers cerebrovascular events as an outcome. The European “SCORE” (Systematic Coronary Risk Evaluation) includes geographic variability within European countries as a calibration metric.
Data from NHANES showed that a Framingham-type risk model was useful in women for predicting cardiovascular events, with a C statistic of 0.829. However, its focus on 10-year risk estimates makes the Framingham Risk Score less useful in younger women. A focus on the lifetime risk for CVD may furnish a better way of assessing risk in women.
Women-specific guidelines recommend stratifying women into three categories—high risk, at risk, and optimal risk—and place emphasis on the lifetime risk for CVD in women (Table 77-1). “High-risk” status is defined as having one or more high-risk states, including the clinical presence of CHD, CVD, peripheral arterial disease (PAD), abdominal aortic aneurysm, end-stage or chronic kidney disease, diabetes mellitus, or 10-year predicted risk for CVD of 10% or greater—a significantly lower threshold for the definition of high risk than the cutoff of 20% used in previous guidelines and the NCEP ATP III guidelines. “At-risk” status is defined as having one or more of the listed risk factors, as well as evidence of advanced subclinical atherosclerosis (e.g., coronary calcification, carotid plaque, or increased intima-media thickness), poor exercise capacity on a treadmill test and/or abnormal heart rate recovery after stopping exercise, systemic autoimmune collagen-vascular disease (e.g., SLE or RA), and a history of preeclampsia, gestational diabetes, or pregnancy-induced hypertension. “Optimal risk” includes total cholesterol lower than 200 mg/dL, blood pressure lower than 120/80 mm Hg, fasting blood glucose lower than 100 mg/dL, BMI lower than 25 kg/m2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree