Ming Hui Chen, Thomas Force
Cardiovascular Complications of Cancer Therapeutic Agents
With advances in modern cancer treatments, patient outcomes have improved substantially, resulting in longer lifespans for these patients. However, with prolonged survivorship, more cancer patients are at risk of developing cardiovascular problems, including heart failure (HF). Indeed, the cumulative incidence of cardiovascular disease at 10 years has been reported as high as 22% in patients who have been treated for malignancy.1 Thus it is becoming increasingly important for cardiologists to be familiar with issues of cardiovascular complications of cancer therapy, as well as to know how to manage these long-term consequences of cancer treatment.
Complicating the situation, drug development in cancer therapeutics has changed more dramatically in the last decade than in any other era. This chapter presents an overview of the cardiovascular toxicity of traditional chemotherapeutic agents and also of the newer targeted therapeutic agents. For the targeted therapeutics, the key message is that each needs to be considered individually in terms of specific targets and mechanisms of action.2 This approach can be expected to lead the field away from the concept of “class effects” of these agents.
Traditional Chemotherapeutic Agents
Commonly used chemotherapy agents are listed in Table 69-1.
TABLE 69-1
Chemotherapeutic Agents Implicated in Clinical Syndromes of Cardiotoxicity
| AGENT | FREQUENCY OF CARDIOTOXICITY* | COMMENTS |
| Left Ventricular Dysfunction–Heart Failure | ||
| Chemotherapeutics | ||
| Anthracyclines | ||
| Doxorubicin | +++ | Highly dose-dependent Risk factors include age (old and young), prior mediastinal radiation, history of heart disease, decreased ejection fraction; drop in ejection fraction on drug therapy, female sex (for children), and other agents (especially trastuzumab) Risk decreased by liposomal encapsulation or dexrazoxane |
| Epirubicin | + | |
| Idarubicin | ++ | |
| Alkylating agents | ||
| Cyclophosphamide | +++ | Primarily seen with high-dose “conditioning” regimens Risk factors are previous mediastinal irradiation and anthracycline drug therapy Also can have myocarditis, pericarditis, myocardial necrosis |
| Ifosfamide | +++ | |
| Taxanes | ||
| Paclitaxel | +/++ | Also employed in paclitaxel-eluting stent |
| Docetaxel | + | |
| Proteasome inhibitor | ||
| Bortezomib | +/++ | Moderately high rates of HF seen in trials (5%), but rates only minimally higher than in patients receiving dexamethasone |
| Targeted Therapeutics | ||
| Monoclonal antibodies | ||
| Trastuzumab | ++ | Not common as single agent Increased risk with anthracyclines, paclitaxel, cyclophosphamide |
| Pertuzumab | +/++ | Targets HER2 Rates of LV dysfunction as high as ~25% in one series |
| Bevacizumab | +/++ | Targets VEGF-A (ligand for VEGFRs) and serves as a trap, preventing interaction with its receptor HF can be seen in setting of severe hypertension, which occurs in 10%-25% of patients, depending on dose; anthracyclines may increase HF risk |
| Tyrosine kinase inhibitors | ||
| Imatinib, nilotinib | + | Can cause severe fluid retention with peripheral edema, pleural and pericardial effusion not secondary to LV dysfunction |
| Dasatinib | ++ | Same as above re fluid retention Can cause severe pulmonary hypertension, mechanism unclear |
| Sunitinib | +++ | LV dysfunction common; hypertension likely plays role |
| Sorafenib | ++ | Rate of cardiotoxicity not clear as of yet |
| Ischemic Syndromes | ||
| Fluorouracil, capecitabine | ++ | ACS; patients with CAD at increased risk Recurs with rechallenge; multiple mechanisms proposed; etiology remains unknown |
| Cisplatin, carboplatin | + | ACS caused by vasospasm or vascular injury Hypertension common; thromboembolism more common (see below) |
| Interferon-α | + | Risk of ischemia increased in patients with CAD; hypertension common |
| Paclitaxel | + | Myocardial ischemia in 1%-5%; serious ischemic cardiac events not common |
| Docetaxel | + | Limited data, but rate probably ~1% |
| Bevacizumab | ++ | Arterial thrombotic events including MI and stroke |
| Vinca alkaloids | + | ~1% risk of cardiac events; ischemia possibly caused by coronary spasm |
| Sorafenib | +/++ | ~2.5% risk of ACS |
| Erlotinib Nilotinib | +/++ | Limited data, but rate ~2% Concern over possible increase in peripheral vascular events |
| Hypertension | ||
| Cisplatin | ++++ | |
| Bevacizumab | ++++ | Extremely common with all anti-VEGF therapeutics to date |
| Sunitinib | ++++ | Intrinsic in mechanism of action of these agents |
| Sorafenib | +++ | |
| Venous Thrombosis | ||
| Cisplatin | +++ | Deep vein thrombosis or pulmonary embolism in 8.5%; most occur early in treatment Additional risk factors for deep vein thrombosis often present |
| Thalidomide | ++++ | Uncommon with monotherapy, but risk rises with concurrent chemotherapy |
| Lenalidomide | +++ | See comments for thalidomide |
| Erlotinib | ++ | Rate with erlotinib plus gemcitabine ~2% over that with gemcitabine alone |
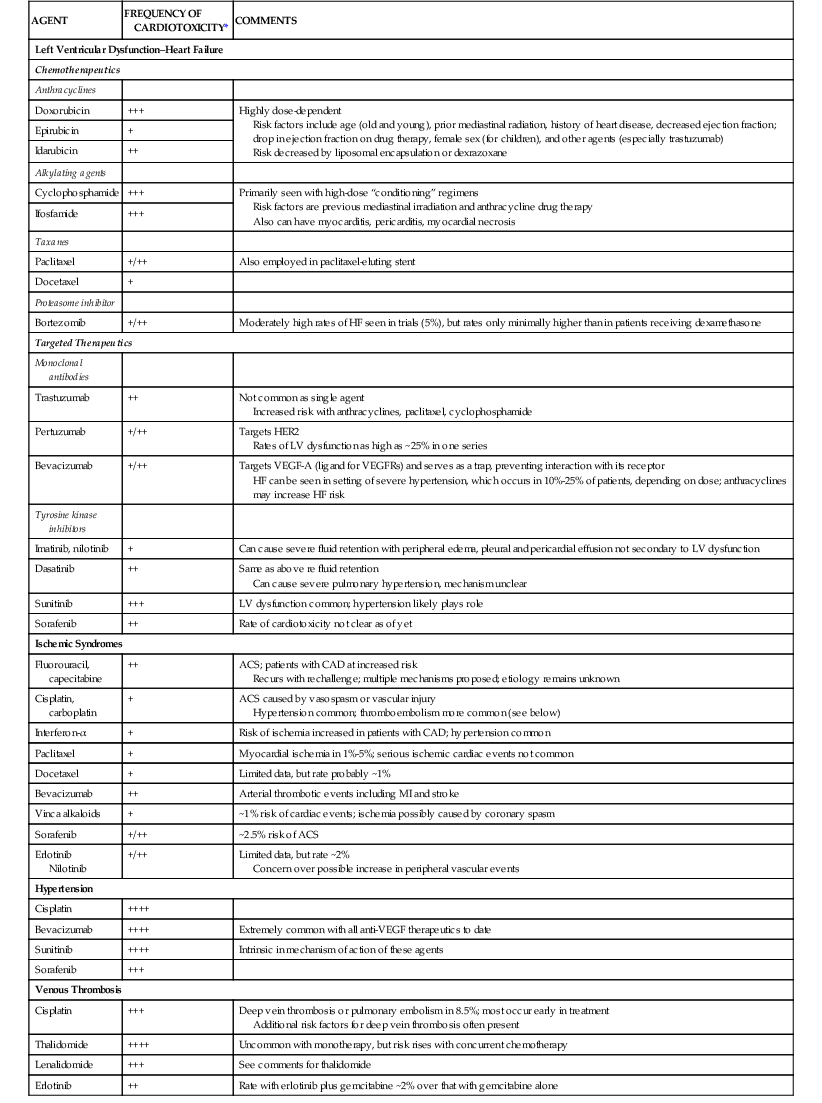
* Relative frequency of cardiotoxicity is scored as follows: + = ≤1%; ++ = 1% to 5%; +++ = 6% to 10%; ++++ = >10%.
ACS = acute coronary syndrome; CAD = coronary artery disease; HER2 = human epidermal growth factor receptor 2; LV = left ventricular; MI = myocardial infarction; VEGF = vascular endothelial cell growth factor; VEGFR = vascular endothelial cell growth factor receptor.
Modified from Yeh ET, Bickford CL: Cardiovascular complications of cancer therapy: Incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol 53:2231, 2009.
Anthracyclines
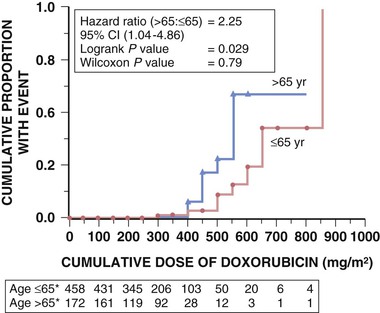
The anthracyclines currently approved in the United States, doxorubicin (Adriamycin), daunorubicin (Cerubidine), epirubicin (Ellence), and idarubicin (Idamycin PFS), and a related compound, mitoxantrone, are key components of many chemotherapeutic regimens, having demonstrated efficacy in lymphomas and many solid tumors, including breast and small cell lung cancer (SCLC).3 This class of agents clearly is the most cardiotoxic to date, acutely producing arrhythmias, left ventricular (LV) dysfunction, and pericarditis, and chronically producing LV dysfunction and HF. The toxicity is strongly dose-related. Initial retrospective analyses suggested that the incidence of HF was 2.2% overall and 7.5% in patients receiving a cumulative dose of 550 mg/m2. More comprehensive analyses, however, have suggested that the incidence is higher than this.4 The incidence rises significantly for cumulative doses above 400 to 450 mg/m2 for doxorubicin (Fig. 69-1). Consequently, with most tumors, oncologists typically limit the dose to 450 to 500 mg/m2.3 If anthracycline cardiomyopathy develops, it often appears within the first year of completion of therapy, with a median of 5 to 9 months, and continues to progress. Anthracycline-induced cardiomyopathy also may be of late onset, and, if so, it typically is chronic.
Risk factors for cardiotoxicity, in addition to doses above 450 mg/m2, include advanced age, history of cardiac disease, and previous mediastinal irradiation (see Fig. 69-1 and Table 69-1). Predictors of cardiotoxicity based on assessment of LV function include a baseline LV ejection fraction (LVEF) less than 50% and a decline in LVEF of more than 10% during treatment to a level less than 50%. Diastolic dysfunction may be the first abnormality noted. Children are particularly susceptible to anthracycline cardiotoxicity; in one series, HF had developed in 5% at 15 years of follow-up, and the incidence increased to 10% for cumulative doses of 550 mg/m2.5 In addition to dose and mediastinal irradiation, age at diagnosis and female sex are predictors for adverse outcomes in children. HF may become clinically evident years after treatment, when these children become adults.6
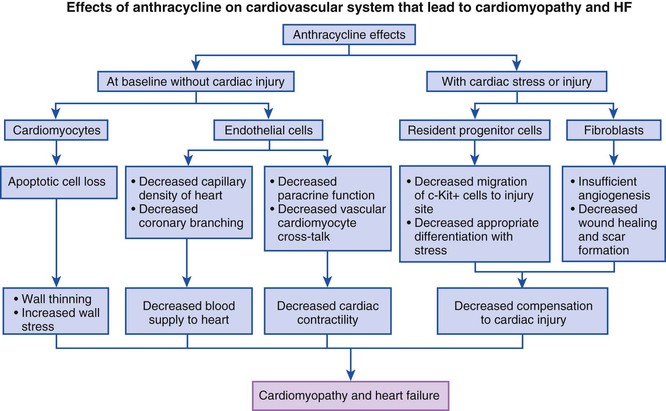
Endomyocardial biopsy is the most sensitive method to detect anthracycline cardiotoxicity, with typical findings of cytosolic vacuolization, lysis of myofibrils, and cellular swelling—features more typical of a necrotic form of cell death. Abnormalities on electron microscopy, however, have not been shown to correlate highly with risk for development of HF and often are present in patients receiving cumulative doses well below those associated with an increased risk of HF. In view of the technical skills required for the procedure and its inherent risks, endomyocardial biopsy is not a practical way to detect or to monitor patients with anthracycline cardiotoxicity, and serial determination of LV function, although insensitive, is the currently accepted method. Recently, the use of biomarkers of injury, most prominently hs-TnI (highly sensitive troponin I), has been reported to identify patients at risk of developing LV dysfunction. In one study, an elevated TnI level after anthracycline therapy was able to predict patients at risk for development of LV dysfunction, and prophylactic angiotensin-converting enzyme (ACE) inhibitor therapy limited LV dysfunction.7,8 Novel imaging strategies such as peak systolic longitudinal strain ultrasound techniques also may prove to be helpful, with benefit additive to that of biomarkers, although more validation is needed before implementation of these strategies in the clinic.9
Other strategies have been used to limit anthracycline cardiotoxicity, including the use of epirubicin, a stereoisomer of doxorubicin. This agent produces less cardiotoxicity than doxorubicin at comparable doses, and 900 to 1000 mg/m2 of epirubicin produces cardiotoxicity comparable to that seen with 450 to 500 mg/m2 of doxorubicin. However, efficacy of the two agents appears to be comparable at equivalent doses. The use of ACE inhibitors was noted previously, but selected studies with angiotensin II receptor blockers and beta blockers also have suggested cardioprotective effects.10 At present, it probably is reasonable to use these therapies prophylactically in high-risk patients until more data are available.
A baseline determination of the patient’s LV function is indicated before initiation of anthracycline therapy, and this parameter should be monitored periodically thereafter, especially when the cumulative dose rises above 300 to 350 mg/m2 for doxorubicin or above comparable doses for the other anthracyclines. The criteria noted earlier concerning risk factors, baseline LV function, and deterioration of LV function, in combination with dosage consideration, can be used for risk-stratification of patients for the development of HF. Use of hs-TnI measurements may improve predictive accuracy and may be particularly helpful in patients receiving high-dose chemotherapy. In those patients, an elevated TnI level predicted subsequent development of LV dysfunction with high sensitivity, albeit low predictive accuracy. A negative TnI assay result strongly predicted the likelihood that LV function would not deteriorate.8 Earlier studies of anthracycline cardiotoxicity have suggested a high mortality rate, but with more modern approaches to the management of patients with HF, the prognosis is better.
Taxanes
The taxanes, paclitaxel (Taxol) and its semisynthetic analogue docetaxel (Taxotere), disrupt microtubular networks as their mechanism of antitumor activity and are effective in breast cancer. Used alone, these drugs have relatively little cardiotoxicity, with occurrence of predominantly asymptomatic bradycardia and, much less often, heart block. However, when paclitaxel was given in close combination with high-dose doxorubicin, high rates of congestive heart failure (CHF) were observed (21%).13 This was found to be secondary to alteration of metabolism of doxorubicin by the taxanes. Further studies demonstrated that doxorubicin doses of less than 380 mg/m2 and separating infusion of paclitaxel by up to 4 hours after doxorubicin administration decreased the risk of HF to less than 5%.14 Docetaxel given with anthracyclines is reported to only mildly increase risk of HF, thought to be secondary to differences in pharmacokinetics and pharmodynamics.13
Alkylating Agents and Antimetabolites
Alkylating agents and antimetabolites are classes of agents generally associated with low incidence of cardiotoxicity (see Table 69-1). Cyclophosphamide (Cytoxan) is relatively well tolerated when it is used at conventional doses, but in patients receiving conditioning regimens before autologous stem cell transplantation, which include high-dose cyclophosphamide, acute cardiotoxicity can occur.15 As opposed to the total cumulative dosage for anthracyclines, dosage in an individual course of treatment is more predictive for cyclophosphamide toxicity. Risk factors include previous anthracycline therapy and mediastinal irradiation and possibly previous imatinib therapy.1 Clinically, patients may present with HF, myocarditis, or pericarditis. In one series of 17 consecutive patients receiving induction therapy, none of whom developed HF, LV dilation as revealed by cardiac magnetic resonance (CMR) was evident from the onset. Mechanisms underlying the toxicity are thought to be injury of both endothelial cells and myocytes, and a picture of hemorrhagic myocardial necrosis can emerge. Patients who survive the acute phase typically do not have residual LV dysfunction. High-dose ifosfamide (Ifex) induced HF in 17% of patients.
Cisplatin (Platinol)-based regimens are the cornerstone of therapy for testicular germ cell cancer, the most common malignant neoplasm in men 20 to 40 years of age; 80% of those with disseminated nonseminoma tumors achieve long-term survival. Thus, in addition to short-term toxicity, long-term toxicity is a concern in this group. Cisplatin is notable for causing hypertension, which is sometimes severe. Acute chest pain syndromes, including myocardial infarction (MI), also have been reported, possibly related to coronary spasm. Because cisplatin often is used in combination with bleomycin, an agent that can induce Raynaud phenomenon in approximately one third of patients, long-term vascular toxicity is particularly a concern. Indeed, after a median 10-year follow-up period after treatment with platinum-based regimens (cisplatin or carboplatin [Paraplatin]) versus radiation therapy, 6.7% of patients in the chemotherapy group and 10% of those in the irradiation group suffered a cardiac event, for a relative risk of 2.4- to 2.8-fold compared with patients treated with surgery only. Changes in the ratio of carotid intima-media thickness were detected as early as 10 weeks after a course of cisplatin-based chemotherapy. The morbidity data have led to calls for more conservative approaches in patients at low risk for cancer recurrence. Nephrotoxicity also is common.
Fluorouracil (Adrucil) is used in the treatment of many solid tumors, and regimens based on this agent have been the mainstay for treatment of colorectal cancer. Fluorouracil can cause acute ischemic syndromes ranging in severity from angina to MI; such syndromes can occur in patients without coronary artery disease (CAD) (approximately 1% of patients), although they are more common in patients with preexisting disease (4% to 5%). Overall, rates range from 0.55% to 8%, although more sensitive methods of detecting possible subclinical ischemia (ambulatory electrocardiographic monitoring) find much higher rates. Discontinuation of treatment with institution of standard antianginal therapies usually leads to resolution of symptoms, but ischemia often recurs if therapy is reinitiated. An alternative agent, capecitabine (Xeloda), is metabolized to fluorouracil, preferentially in tumor cells, suggesting that it may have less cardiotoxicity. However, one retrospective review (that excluded patients with “significant” cardiac disease) found an incidence of 6.5% for major cardiac events for the combination of capecitabine and oxaliplatin that included angina (4.6%), MI, ventricular tachycardia, and sudden death.1 Vasospasm is thought to be the mechanism triggering ischemia, although thromboembolic events also are increased. Not clear at this point is whether prophylactic nitrates prevent ischemic events. Capecitabine monotherapy appears to be associated with a lower incidence of cardiac toxicity than fluorouracil monotherapy.
Proteasome Inhibitors
Bortezomib (Velcade) is an inhibitor of the proteasome system responsible for degrading improperly folded proteins and proteins that are no longer needed in the cell. The drug is approved for use in patients with multiple myeloma. The concept behind its use is that malignant cells have altered proteins regulating the cell cycle, leading to more rapid cell division and increased accumulation of damaged proteins as a result. Therefore the continued health of the malignant cell, as opposed to normal cells, may be more dependent on degradation of the damaged proteins. In support of this concept, proteasome inhibitors are more toxic to proliferating malignant cells in culture than to normal cells. Targets include activation of endoplasmic reticulum stress pathways leading to activation of proapoptotic factors and inactivation of survival factors. Cardiomyocytes have an active proteasome system, raising concerns that inhibitors may be cardiotoxic. However, in the phase III trials of bortezomib, HF developed in 5% of treated patients but also in 4% of patients in the dexamethasone arm of the trial.16 Higher rates have been reported, however, and mice treated with the agent exhibited significant LV dysfunction, ultrastructural abnormalities, and mitochondrial dysfunction.17
Other Agents
The cardiotoxicity of additional chemotherapeutic agents, including cytokines (interleukin-2 [IL-2], aldesleukin [Proleukin], and denileukin diftitox), interferons, and suberoylanilide hydroxamic acid, as well as topoisomerase inhibitors (etoposide and teniposide), purine analogues (pentostatin and cladribine), all-trans retinoic acid (ATRA), arsenic trioxide, and thalidomide and lenalidomide, is discussed in the online supplement for this chapter (Cardiovascular Complications of Additional Cancer Therapeutic Agents).![]()
Cardiovascular Complications of Additional Cancer Therapeutic Agents
Additional agents that can cause cardiotoxicity include cytokines (IL-2, aldesleukin [Proleukin], and denileukin diftitox) and interferons, HDAC inhibitors (trichostatin A and suberoylanilide hydroxamic acid), topoisomerase inhibitors (etoposide and teniposide), purine analogues (pentostatin and cladribine), all-trans retinoic acid (ATRA) (Tretinoin), arsenic trioxide, and thalidomide/lenalidomide.
All-Trans Retinoic Acid
ATRA (Tretinoin) is used primarily in the treatment of acute promyelocytic leukemia (APL). APL is caused by a translocation that creates the promyelocytic leukemia–retinoic acid receptor-α fusion protein (PML-RARα). This inactivates RARα, and leads to active suppression of genes that favor differentiation of promyelocytes to granulocytes.1 ATRA leads to the dissociation of PML-RARα from target genes, releasing them from repression and allowing differentiation of the leukemic promyelocytes into mature myeloid cells. However, 5% to 27% of patients develop retinoic acid syndrome, characterized by fever, respiratory distress, interstitial pulmonary infiltrates, pulmonary edema, pleural and pericardial effusions, hypotension, and, in approximately 10% of cases, acute renal failure or CHF.2 Between 5% and 29% of these patients die, with the lower rates due in part to more aggressive use of corticosteroids in more recent series of patients.2 Mechanisms underlying the syndrome are not clear but are thought to involve microvascular damage.
Cytokines
The cytokines IL-2 and aldesleukin and the IL-2 receptor are targets of therapy. IL-2 is approved for use in patients with metastatic renal cell carcinoma and melanoma. Its mechanism of action is thought to be based on its ability to expand lymphocyte populations and to increase effector functions of these cells, thereby limiting tumor growth.3 Infusions can rarely lead to a syndrome resembling septic shock with hypotension and vascular leak syndrome, and rarely, MI or myocarditis may develop.4 Another strategy involves “loading” IL-2 with diphtheria toxin (denileukin diftitox [Ontak]), thereby delivering the toxin to cells expressing the IL-2 receptor. This agent, which has been approved for cutaneous T cell lymphoma, also can cause vascular leak syndrome (in 27% of patients in one study), but steroid premedication is effective at preventing or reducing the severity of this complication.5 Thrombotic events may occur in up to 10% of patients.
Interferons are a large family of cytokines that can induce many types of cancerous cells to undergo apoptosis. Interferon-α (available in a number of formulations) is approved for use in many malignancies. Infusion of these agents can lead to a wide range of clinical manifestations of toxicity, generally appearing within the first few hours after treatment in approximately 10% of patients. Flulike symptoms, tachycardia, and either hypotension or hypertension can occur. Ischemia and MI are rare but occur with increased frequency in patients with CAD. Treatment is supportive, and symptoms are not long-lasting.
Topoisomerase Inhibitors
Topoisomerase types I and II transiently cleave and then religate DNA. Type I topoisomerase cleaves single-stranded DNA, and type II topoisomerase cleaves double-stranded DNA. Topoisomerase inhibitors stabilize the DNA-enzyme complex in its cleaved form, producing DNA single- or double-strand breaks that trigger cell death. Although anthracyclines are topoisomerase II inhibitors, this mechanism appears to have little or nothing to do with anthracycline cardiotoxicity. Thus it was not clear whether newer topoisomerase II inhibitors, such as the epipodophyllotoxins, etoposide (VePesid, Etopophos, Toposar) and teniposide (Vumon), or the aminoacridine amsacrine (m-AMSA), would also have cardiotoxic effects. Etoposide can lead to hypotension, but ischemia and MI, although very rare, have been reported. Patients who have received previous chemotherapy or mediastinal irradiation may be at slightly greater risk for MI. Amsacrine (approved in Canada) interacts with the human ether-à-go-go–related [gene] (HERG) channel, mutations in which cause hereditary long-QT syndrome. Amsacrine can produce significant QT prolongation, torsades, and ventricular fibrillation.6 It is not clear whether topoisomerase I inhibitors (e.g., irinotecan [Camptosar]) are cardiotoxic.
Purine Analogues
The purine analogues pentostatin (Nipent) and cladribine (Leustatin) are used primarily in the treatment of hairy cell leukemia. Thee agents interact with adenosine deaminase, a key enzyme in purine metabolism that is present in high amounts in lymphoid cells. Cardiotoxicity is rare, but manifestations can include atrial arrhythmias and MI. Caution should be exercised in treating elderly patients with cardiovascular disease. The combination of high-dose purine analogue and cyclophosphamide may somewhat increase risk of cardiotoxicity.
Thalidomide and Lenalidomide
Thalidomide (Thalomid) and lenalidomide (Revlimid) are both antiangiogenic and immunomodulatory and are used in the treatment of multiple myeloma and myelodysplastic syndromes.7 Mechanisms of action are complex, but in addition to blocking angiogenesis, these agents inhibit production of interleukin 6 (a growth factor for myeloma cells) and activate apoptosis.8 They also augment natural killer cell–dependent cytotoxicity. Cardiac toxicity is rare with these agents, but peripheral edema and sinus bradycardia have been reported. Risk of deep vein thrombosis is thought to be low with monotherapy but is increased when either drug is used in combination with anthracyclines.9 Owing to the high incidence of severe birth defects with thalidomide that were identified in the 1960s, female patients are followed closely with routine pregnancy testing if they are of childbearing age.
Arsenic Trioxide
Arsenic trioxide (Trisenox) is used in the treatment of the 20% to 30% of patients with acute promyelocytic leukemia who relapse after ATRA and anthracycline therapy. This agent induces partial differentiation and also apoptosis of the leukemic cells. Its major adverse effect on the heart is prolongation of the QT interval, and QTc (corrected QT interval) longer than 500 milliseconds was noted on at least one electrocardiographic tracing in 40% of patients.10 The prolongation is reversible but has been associated with torsades de pointes and sudden death, occurring within the first 24 hours after therapy. However, with careful monitoring of patients, correction of hypokalemia or hypomagnesemia, and avoidance of other drugs that prolong the QT interval, arsenic trioxide can be administered safely.10