Essentials of Diagnosis
General Considerations
Cardiogenic shock is an extremely morbid condition. Despite recent advances in treatment, nearly 50% of patients with cardiogenic shock still do not survive to hospital discharge. Cardiogenic shock develops as a result of the failure of the heart in its function as a pump, resulting in inadequate cardiac output. This failure is most commonly caused by extensive myocardial damage from an acute myocardial infarction (MI), but other mechanical complications of an acute MI, valve lesions, arrhythmias, and cardiomyopathies can also lead to cardiogenic shock.
Definition
Cardiogenic shock is defined by both the clinical signs of a reduced cardiac output and associated hemodynamic findings. Clinical signs of reduced cardiac output include cool extremities, weak distal pulses, altered mental status, and diminished urinary output (> 30 mL/h). Hemodynamic findings in cardiogenic shock include a reduced cardiac output without evidence of hypovolemia. One commonly used set of hemodynamic criteria are as follows: (1) a systolic blood pressure of < 90 mm Hg for at least 30 minutes (or the need for medications or devices to maintain a systolic blood pressure ≥ 90 mm Hg), (2) a pulmonary capillary wedge pressure (PCWP) of > 15 mm Hg (which excludes hypovolemia), and (3) a cardiac index < 2.2 L/min/m2.
Etiology
Acute MI accounts for most cases of cardiogenic shock. Acute MI results in cardiogenic shock in 5–10% of patients presenting for emergency care; however, it is likely that cardiogenic shock develops in many more patients following an acute MI, but they do not survive to receive medical attention. Cardiogenic shock may occur in a patient with a massive first infarction, or it may occur with a smaller infarction in a patient with a weakened heart from prior MIs. “Mechanical” complications of an acute MI can also cause shock, and these include ventricular septal defect (VSD), acute mitral regurgitation as a result of papillary muscle rupture, and myocardial free wall rupture with tamponade. Right ventricular infarction in the absence of significant left ventricular infarction or dysfunction can lead to shock. Refractory tachyarrhythmias or bradyarrhythmias, usually in the setting of preexisting left ventricular dysfunction, are occasionally a cause of shock and can occur with either ventricular or supraventricular arrhythmias. Cardiogenic shock may occur in patients with end-stage cardiomyopathies (ischemic, valvular, hypertrophic, restrictive, or idiopathic in origin). Cardiogenic shock may also be the presenting manifestation of acute myocarditis (infectious, toxic, rheumatologic, or idiopathic). A more recently recognized entity is stress cardiomyopathy (also known as apical ballooning syndrome or takotsubo cardiomyopathy) in which severe heart failure and sometimes cardiogenic shock result from extreme emotional distress. Finally, certain endocrine abnormalities may cause severe cardiac dysfunction and cardiogenic shock (Table 9–1).
I. Acute myocardial infarction (MI) A. Pump failure B. Mechanical complications of acute MI 1. Acute mitral regurgitation 2. Ventricular septal defect 3. Free wall rupture/tamponade C. Right ventricular MI II. End-stage, severe cardiomyopathies secondary to A. Valvular disease B. Chronic ischemic disease C. Restrictive/infiltrative D. Idiopathic III. Acute myocarditis: viral/infectious, toxic IV. Stress cardiomyopathy V. Endocrine disease (eg, hypothyroidism, pheochromocytoma) A. Bradyarrhythmias B. Tachyarrhythmias VI. Secondary to medications VII. Posttraumatic |
Pathogenesis
The principle feature of shock is hypotension with evidence of end-organ hypoperfusion. In cardiogenic shock, this occurs as a consequence of inadequate cardiac function. The usual response to low cardiac output is sympathetic stimulation to increase cardiac performance and maintain vascular tone. This results in tachycardia and increased myocardial contractility (β-adrenergic–mediated effects) and peripheral vasoconstriction (an α-adrenergic–mediated effect). The classic patient with cardiogenic shock has evidence of peripheral vasoconstriction (cool, moist skin) and tachycardia. Corresponding typical hemodynamics are a reduced cardiac output and increased systemic vascular resistance (SVR), defined as:
Recent evidence suggests that many patients with cardiogenic shock do not have these typical hemodynamics and instead have a lower SVR much like patients in septic shock. In fact, it has been postulated that a systemic inflammatory response–like syndrome with a low SVR may be encountered in up to 25% of patients in cardiogenic shock. Furthermore, patients with severe septic shock often have depressed myocardial function, and patients with cardiogenic shock can have a component of hypovolemia. Thus, there can be considerable overlap in pathophysiologies.
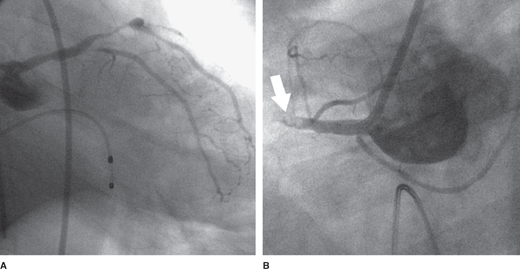
If at least 40% of the left ventricular myocardial muscle mass is lost, either acutely or as a result of prior damage, cardiogenic shock can result from pump failure (ie, there is not sufficient left ventricular muscle mass to maintain forward cardiac output). This usually occurs as a consequence of an MI. The initial event in an acute MI is obstruction of a coronary artery, commonly termed the “infarct-related artery.” The acute obstruction decreases oxygen supply to a portion of the heart, resulting in myocardial ischemia and infarction, which in turn leads to diminished myocardial contractility. The ensuing drop in cardiac output and blood pressure leads to decreased perfusion pressures in other coronary beds. (Coronary perfusion becomes compromised when the aortic diastolic pressure falls below 50–55 mm Hg.) This results in further ischemia, especially if stenoses are present in these non–infarct-related vessels, and additional deterioration in left ventricular function occurs. Indeed, most patients with shock after acute MI have extensive coronary disease, and mortality correlates with the extent of coronary disease (Figure 9–1).
The process of ischemia and infarction leading to myocardial dysfunction leading to further ischemia, and so on, has been appropriately termed “a vicious cycle.” Evidence for this vicious cycle is found in autopsy studies that show infarct extension at the edges of an infarct in addition to discrete, remote infarctions throughout the ventricle. This also explains the finding that cardiogenic shock can occur immediately, provided sufficient myocardium is dysfunctional, or occur hours after the initial infarct as a consequence of this vicious cycle. Tissue hypoperfusion also leads to accumulation of lactic acid. Acidemia is detrimental to left ventricular contractility, and this is another example of a vicious cycle contributing to the pathophysiology of cardiogenic shock.
The pathophysiology of cardiogenic shock due to mechanical complications of acute MI is somewhat different. The three main mechanical problems are (1) acute mitral regurgitation as a consequence of papillary muscle rupture, (2) VSD, and (3) myocardial free wall rupture leading to cardiac tamponade. These mechanical problems all occur in a bimodal distribution, with some occurring earlier in the presentation and others occurring later, and are a consequence of weakened, necrotic myocardium.
The papillary muscles anchor the mitral valve apparatus to the left ventricle. Proper papillary muscle function is vital in ensuring that the two mitral valve leaflets close completely to prevent leakage or regurgitation of blood backward into the left atrium. Papillary muscle rupture is a term used somewhat erroneously; rupture and avulsion of the entire papillary muscle usually result in such severe regurgitation that it is rapidly fatal. If only a portion of the papillary muscle ruptures, then severe mitral regurgitation ensues, leading to pulmonary edema and a reduced forward cardiac output. This accounts for up to 7% of patients with cardiogenic shock after an acute MI. The sympathetic nervous system response to cardiac failure results in increased SVR (afterload) and a further increase in the regurgitant fraction, another example of a vicious cycle contributing to cardiogenic shock.
Rupture of the myocardial free wall results in bleeding into the relatively nondistendible pericardial space and leads rapidly to pericardial tamponade and cardiovascular collapse. Often this is immediately fatal, but occasionally, patients survive and cardiogenic shock develops. The incidence of free wall rupture in patients with cardiogenic shock is as high as 3%.
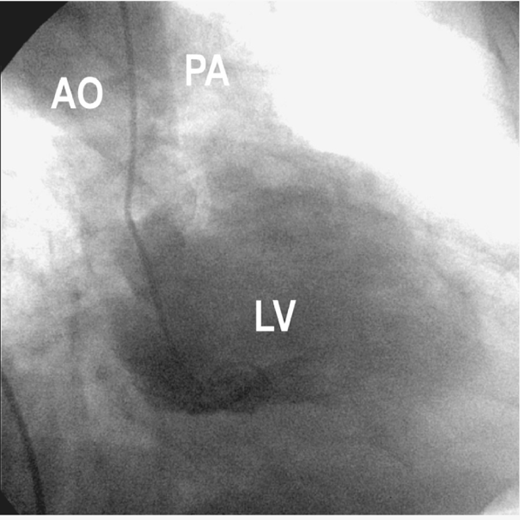
Rupture of the intraventricular septum with the formation of a VSD has an incidence of approximately 0.3% in patients with acute MI and accounts for up to 6% of patients with cardiogenic shock after an acute MI. A large VSD causes significant shunting of blood from the left ventricle to the right ventricle and results in right ventricular volume and pressure overload (Figure 9–2). Shock usually develops as a consequence of reduced forward cardiac output. As with acute mitral regurgitation, the sympathetic nervous system response results in increased afterload, thereby shunting an even larger fraction of the cardiac output across the interventricular septum.
Right ventricular infarctions occur in approximately 40% of patients with inferior MIs. Right ventricular infarctions may result in cardiogenic shock without significant left ventricular dysfunction. Failure of the right ventricle leads to diminished right ventricular stroke volume, which results in a decreased volume of blood returning to the left ventricle. This markedly diminished left ventricular preload, even with normal left ventricular contractility, causes a decreased systemic cardiac output. The right ventricle also becomes dilated, which results in displacement of the intraventricular septum to the left. If severe, this can actually impair left ventricular filling, with physiology similar to that seen in cardiac tamponade. Since left ventricular filling pressures are not elevated in pure right ventricular failure, pulmonary congestion will not be evident.
A variety of arrhythmias can contribute to the development of shock. A sustained arrhythmia, that is, one that does not culminate in ventricular fibrillation and sudden death, is generally a cause of shock only in the already compromised ventricle. Atrial and ventricular tachyarrhythmias can result in diminished time for ventricular filling in diastole as well as the loss of the atrial contribution to ventricular diastolic filling. This results in a diminished preload, which in turn results in a decreased stroke volume. These factors may be enough to result in cardiogenic shock in patients with already impaired left ventricular function or with conditions such as severe aortic stenosis in which the left ventricle is especially sensitive to filling pressures. Bradyarrhythmias reduce cardiac output as a consequence of the slow heart rate. Because total cardiac output is a function of heart rate and stroke volume (cardiac output = stroke volume × heart rate), a markedly decreased heart rate, especially with concomitant left ventricular dysfunction may, result in shock.
Many forms of heart disease can result in an end-stage dilated cardiomyopathy. These patients may be in such acutely decompensated states that they are in frank cardiogenic shock.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree